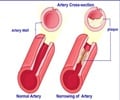
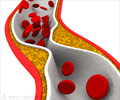
Indian pharmaceutical company Ranbaxy Laboratories confirmed that it will be stopping the production of its generic version of cholesterol drug Lipitor.
While there have been no reports of any harm caused to its users, the US Food and Drug Administration revealed that Ranbaxy had informed that it will be ceasing the production of the drug until its investigation is complete.
“Ranbaxy decided to stop making atorvastatin until the company has thoroughly investigated the cause of the contamination and remedied the problem. At this time, we have not received any reports of patient harm due to glass particulates that may be in the recalled product”, the FDA statement read.
Source-Medindia