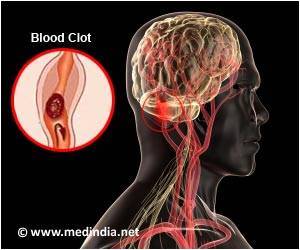
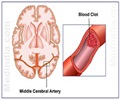
From the onset of stroke symptoms, there is only a 3 to 4 1/2 hour window to use clot-busting drugs to try to restore blood supply to the brain.

‘Up to 80 percent of strokes can be prevented. People at risk for stroke include those who have high blood pressure, high cholesterol, diabetes, and those who smoke.’





The study could offer hope to patients who miss the 6-hour treatment window and allow doctors to offer more patients life-saving neuroendovascular surgery. No randomized trial has previously demonstrated the effectiveness of mechanical thrombectomy for acute stroke caused by a large vessel occlusion (LVO) performed more than 6 hours after the onset of a stroke. "The results of this trial offer tremendous promise of reduced disability to patients who can still be treated after the 6-hour window and for whom until now there were no other evidence based treatment options," said co-principal investigator Dr. Tudor Jovin, from the University of Pittsburgh Medical Center.
The study, DAWN in full daylight (DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention, compared patient outcome 90 days after a thrombectomy using the Trevo® Retriever and medical management or after medical management alone.
Patients were considered for the trial if they had a stroke that began within 6 to 24 hours, or had an unknown time of onset (such as stroke discovered upon waking). Researchers also considered a patient's age, the severity of their stroke symptoms and whether the patients had a clinical core mismatch: a small area of damaged brain tissue but a larger area threatened but still alive.
The trial included 206 patients before enrollment was stopped at the recommendation of the Data Safety Monitoring Board after the study passed pre-specified probability thresholds for predicted success.
Advertisement
Source-Eurekalert