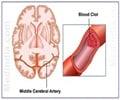
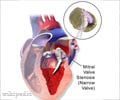
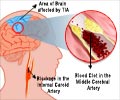
Xarelto, a Bayer-made drug approved in July for preventing blood clots, is so far unproven for a new proposed use as a stroke preventer, US regulators have said.
An advisory committee to the FDA would further discuss the latest findings on Thursday.
"This review posted today has to do with the application for the new use and at this point does not impact the already-approved use," said FDA spokeswoman Sandy Walsh.
Xarelto is currently on the market to reduce the risk of blood clots, deep vein thrombosis, and pulmonary embolism following knee or hip replacement surgery.
Bayer's stock plunged nearly 12 percent by midday in US trading.
Source-AFP