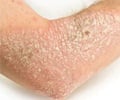
Phase 3 trials conducted on Remicade (infliximab) showed rapid, significant improvement in patients with moderate to severe plaque psoriasis.
Phase 3 trials conducted on Remicade (infliximab) showed rapid, significant improvement in patients with moderate to severe plaque psoriasis. The stud mainly used placebo- control and dose-ranging from 3 mg/kg and 5 mg/kg. The continuous eight week maintenance therapy resulted in greater long-term skin clearance compared with as-needed therapy regimens within each dose.
At 10 weeks it was seen that 70 % of the patients treated with Remicade 3 mg/kg and 75 % of patients receiving 5 mg/kg achieved at least 75 % improvement in psoriasis. The measurement scale used was Psoriasis Area Severity Index (PASI 75). At the end of the 50 weeks patients receiving Remicade 5 mg/kg every eight-week maintenance therapy saw the highest level of improvement. The results were presented at the 64th annual American Academy of Dermatology meeting.Alan Menter, MD, chairman, Division of Dermatology, Baylor University Medical Center, and lead study investigator said that patients receiving scheduled Remicade maintenance therapy achieved long-term cure against psoriasis, a lifelong, chronic inflammatory disease. Statistics show that nearly two million Americans suffer from this disease.
Robert Matheson, MD, Oregon Medical Research Center, and study investigator said that Remicade in the treatment of a disease offers great physical and emotional challenges. It has a potential to result in complete skin clearance.
The United States Food and Drug Administration (FDA) accepted the use of Remicade in the treatment of moderate to severe plaque psoriasis. Hence the European Commission also approved Remicade for the treatment of moderate to severe plaque psoriasis in adults who failed to respond to other systemic therapy.