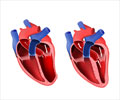
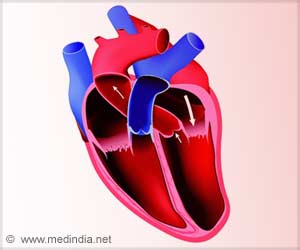
A redefinition of the term cardiac hypertrophy which is currently used to describe changes to the morphology (structure) of the heart is being called for
The term myocardial hypertrophy, say the working group, lacks precision. In cell biology the term hypertrophy describes growth via cell enlargement as opposed to growth by cell division, where hyperplasia is the correct term. Currently in cardiology the term hypertrophy is commonly applied to the situation in the whole heart where myocardial enlargement is accompanied by both hypertrophy and hyperplasia. In addition the term hypertrophy does not take account of the fact that non myocytes in the heart (such as fibroblasts) are not passive bystanders and also change in number when the heart remodels. Additionally there may be an invasion of inflammatory cells into the heart, and angiogenesis (the growth of new blood vessels) may occur.
"The advantage of the term cardiac remodelling is that it simply defines reorganisation of the different cardiac tissue components, and can be used to describe an increase, or decrease in the size of the left ventricle, as well as a change to the cellular components," explains the first author of the paper, Ralph Knöll from the National Heart & Lung Institute, Imperial College (London, UK).
The term "hypertrophy", propose the working group, should in future be restricted to cardiac myocytes (heart muscle cells) rather than the whole heart. The adult mammalian cardiac myocyte is an asymmetric cell that is cylindrical in shape in which myofibrils (composed of repeating sarcomeric units) are aligned along the axis of the cells. Pressure overload causes the addition of sarcomeres in parallel with the long axis of the cell causing a disproportionate increase in cell width; while volume overload causes the addition of sarcomeres in series with the long axis causing a disproportionate increase in cell length.
In the paper the authors challenge the widely held view that any form of cardiac remodelling is ultimately malign, and stress that there are fundamental differences between maladaptive remodelling that leads to heart failure and adaptive remodelling that does not result in heart failure.
"One of the major research tasks is to understand more about these two types of remodelling and to find ways of translating maladaptive remodelling into adaptive remodelling so we can prevent or reverse the course towards this adverse phenotype ," says Knöll. The great potential of experimental models to help investigators gain information on the genetic and molecular events controlling cardiac remodelling is acknowledged by the position paper. Animal models of haemodynamic overload (pressure overload by constriction of the transverse aorta), infarction (by coronary artery ligation), and heart failure (induced by rapid ventricular pacing) are helping to define the processes that result in cardiac remodelling. Furthermore transgenic mice carrying mutations that mimic those responsible for human forms of hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM) are further elucidating the biological changes. Non-invasive imaging techniques, such as echocardiography and magnetic resonance imaging as well as invasive techniques such as heart catheterization, are now allowing investigators to closely follow the development of the pathological phenotype, which helps in the development of new therapeutic strategies. The paper outlines proposed parameters that need to be evaluated to ensure accurate evaluation of the experimental cardiac phenotypes.
Advertisement
The paper concludes with suggestions for an algorithm to define a pathway for investigation of cardiac remodelling in both animal models and patients. The algorithm takes into account whether left ventricular (LV) internal size is changed, LV wall thickness altered, LV basal function altered (contractions of the myocardium) and whether the patient experiences stress intolerance (where the contractile performance does not increase with the injection of catecholamines). "The algorithm is intended to help investigators and clinicians accurately evaluate heart failure phenotypes for better risk stratification and tailored treatment" explains Knöll. "The working group believes that this could be an important tool in future prospective studies for the establishment of new treatment protocols."
Advertisement
Source-Eurekalert