In females, estrogen interferes with the protein channel complex,worsening the effects of CF and leading to higher mortality rates and shorter a lifespan.
‘The findings can be used in the development of new treatment strategies aimed at preventing or reversing’





The gene mutation causes an aberrant form of a protein known as the cystic fibrosis transmembrane conductance regulator (CFTR). Individuals with only one defective copy of the gene are generally disease-free, but may act as carriers of the recessive trait.It is generally recognized that females are more susceptible to the ravages of CF, often displaying more severe symptoms and shorter life expectancy. The hormone estrogen is believed to be a crucial contributing factor to the observed gender gap in CF cases.
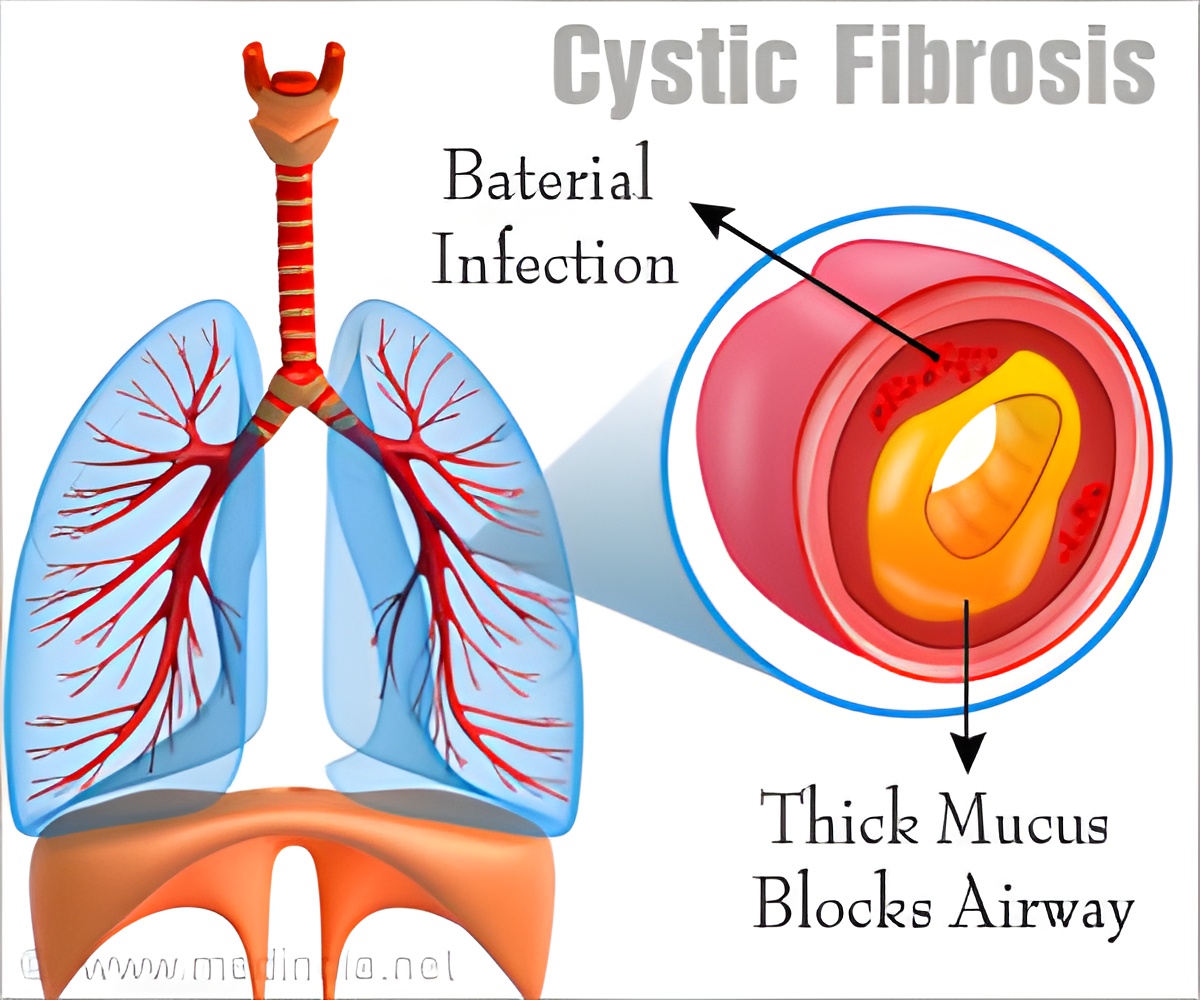
Disruption of ion flux accounts in part for symptoms of CF, which include the ramped-up production of sweat, mucus, and digestive fluids. The condition remains incurable. The vast majority of cystic fibrosis patients die from bacterial infections, particularly from the pathogen pseudomonas.
A number of telltale symptoms of CF include salty-tasting skin, poor growth and poor weight gain and the accumulation of thick, sticky mucus. The patient is often short of breath and bacterial chest infections are common.
Such symptoms typically appear in infancy and childhood. Failure of proper growth may be due to a combination of factors, including chronic lung infection, poor absorption of nutrients through the gastrointestinal tract, and increased metabolic demand as a result of chronic illness.
Advertisement
The research offers new insights into the underlying mechanisms that affect the disease and sets the stage for development of new treatment strategies aimed at preventing or reversing its calamitous effects.
Advertisement
The transport of ions is a vital feature of living systems, allowing a range of physiological processes including heartbeat, hearing, and muscle movements. Defects in ion transport are implicated in a broad range of serious diseases, including cholera, pulmonary edema, and CF.
"One of the most common ways for cells to communicate is through the flux of ions like sodium, potassium, and magnesium. Ion channels are effectively holes in the membrane that are tightly regulated--they're protein-based," Van Horn says.
The ion transport system investigated in the current research involves a critical protein known as KCNE3, which helps properly regulate ion transport. KCNE3 forms a complex with a partner protein-- KCNQ1, which acts as a channel for ions. Together, they form a finely-tuned gateway, permitting or blocking ion transport in order to maintain salt and fluid homeostasis.
Among its many functions, KCNE3 regulates the flow of potassium (K+) ions which impact transport of chloride (Cl-) ions across epithelial tissues. In patients with CF, the efficient migration of chloride ions is impaired. The research provides new insights into the proper functioning of the chloride ion gateway and mechanisms underlying its dysfunction in patients with CF.
"This is an unusual case in biology where this accessory membrane protein binds to the ion channel and effectively turns the ion channel on. That's important especially in the trachea and epithelial tissues in your lungs," Van Horn says.
A better understanding of the structure and function of the ion gateway reveals how estrogen might interfere with the KCNE3-KCNQ1 channel complex, apparently worsening the effects of CF lung disease and leading to higher mortality rates and shorter a lifespan for female patients.
"The focus of this study is to understand how this KCNE3 protein interacts with the ion channel KCNQ1 and how KCNE3 causes the channel to become fully open, or conductive, to ion flow," Van Horn says.
By combining a variety of experimental approaches including structural biology, electrophysiology, cell biology and computational biology, the study reveals the detailed workings of the membrane protein complex, using the predictive power of structural biology to see how estrogen may influence the disruption the channel.
Among the tools used for these investigations is nuclear magnetic resonance (NMR), a technology closely related to the magnetic resonance imaging (MRI) now common in clinical settings for observing tissues and organs in the body with high resolution.
NMR exploits the magnetic properties of atomic nuclei, allowing researchers like Van Horn to zero in on structural details of biomolecules, (including membrane proteins), with remarkable clarity. Using the technique, scientists are able to predict the effects of certain mutations on protein structure and dynamics and optimize drug interactions with target proteins.
It is believed that proper functioning of the KCNQ1 channel helps mitigate fluid buildup in the lungs. When inhibited by high concentrations of estrogen, ion flow is impaired, allowing bacterial infections to take hold. Around 80% of the deaths caused by CF are the result of lung problems. Serious infections caused by the disease are typically treated with antibiotics, which have somewhat improved the prognosis for CF patients, though the disease remains a leading killer.
As Van Horn emphasizes, membrane proteins, like those examined in the current study, account for nearly a third of all human proteins and are key factors in health and disease. Such proteins are targets of the vast majority of drugs and other therapeutic agents, yet a more thorough understanding of their detailed structure and subtle interplay is necessary before CF and a host of other protein-based diseases may be adequately addressed.
"This is really the golden era for the field of membrane protein structural biology. We are starting to really understand how these proteins are working together and what the architectures look like," Van Horn says. "In the long term, a better understanding of how membrane proteins work will help to make CF and other diseases more easily treatable."
Source-Medindia