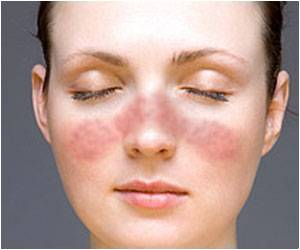
A new study has demonstrated positive results using active immunotherapy against the signaling protein interferon alpha in the treatment of lupus

Interferon alpha (IFNa) is a protein (called a cytokine) made by cells of the immune system, which stimulates the production of autoantibodies in people with lupus. This stimulation of autoantibodies causes an attack on a person''s immune system, and blocking cytokines is a potential target in the treatment of people with lupus.
Immunization to stop the effects of cytokines has already been proven beneficial in animals with lupus, and - for the first time - researchers have looked at an immunization strategy with INFa-kinoid in a study of people with lupus. In the IFNa-kinoid, the structure of the IFN cytokine is artificially modified by linking it to another molecule, called a carrier. This modified cytokine is then ‘seen'' by the immune system, and the immune system starts making antibodies against cytokines - ideally blocking their harmful effects in the body.
"Based on the pivotal role played by Type-I IFN (including IFNa) in the mechanisms underlying lupus, targeted therapy directed against this molecule might reduce the signs and symptoms of lupus, says the study''s lead investigator, Frédéric Houssiau, MD, PhD; head of the Rheumatology Department and full professor of Rheumatology at Université catholique de Louvain, Brussels, Belgium. "The objective of our study was to demonstrate the safety and the feasibility of a new approach that uses the patient''s own immune system against their IFNa."
Twenty-eight patients with mild to moderate, seropositive lupus were enrolled in the study, and the researchers looked at four dosage levels (30, 60, 120 and 240 milligrams) of INFa-kinoid, which were injected directly into the muscle on days zero, seven, 28 and (optionally) 84.
The researchers looked for any negative effects, as well as blood and biochemical responses that might occur with each dose. Responses in the immune system were assessed by using several different scoring systems that evaluate the degree of lupus disease activity.
Advertisement
All participants who received the immunization demonstrated an anti-IFNa antibody response, and these antibodies peaked and then declined after the last immunization was received.
Advertisement
The next step, according to Dr. Houssiau, is to design and to perform a trial to evaluate the drug''s effectiveness.
The American College of Rheumatology is an international professional medical society that represents more than 8,000 rheumatologists and rheumatology health professionals around the world. Its mission is to advance rheumatology. The ACR/ARHP Annual Scientific Meeting is the premier meeting in rheumatology. For more information about the meeting, visit www.rheumatology.org/education. Follow the meeting on Twitter by using the official hashtag: #ACR2011.
Editor''s Notes: Frederic A. Houssiau, MD, PhD will present this research during the ACR Annual Scientific Meeting at McCormick Place Convention Center at 2:45 PM on Tuesday, November 8 in Hall F2. Dr. Houssiau will be available for media questions and briefing at 8:30 AM on Tuesday, November 8 in the on-site press conference room, W 175 C.
Learn more about living well with rheumatic disease as well as rheumatologists and the role they play in health care. Also, discover the ACR''s Simple Tasks campaign, which highlights the severity of rheumatic diseases and the importance of early and appropriate referral to a rheumatologist.
Presentation Number: 27470
Active Immunization Against IFNα with IFN-Kinoid in SLE Patients Is Safe, Immunogenic and Induces Down-Regulation of IFN-Mediated Genes
Frédéric A. Houssiau (Université catholique de Louvain, Brussels, Belgium)
Raskov Rashkov (MHAT Sveti Ivan Rilski, Sofia, Bulgaria)
Eric Hachulla (Internal Medicine, Lille CEDEX, France)
Estibaliz Lazaro (Hôpital Haut-Lévêque, Pessac, France)
Christian Jorgensen (Hospital Lapeyronie, Montpellier, France)
François Spertini (CHU Vaudois, Lausanne, Switzerland)
Xavier Mariette (Université Paris-Sud, Le Kremlin Bicetre, France)
Géraldine Grouard-Vogel (NEOVACS SA, Paris, France)
Bernard Fanget (NEOVACS SA, Paris, France)
Olivier Dhellin (NEOVACS SA, Paris, France)
Bernard Lauwerys (Université catholique de Louvain, Brussels, Belgium)
Pierre Vandepapelière (NEOVACS SA, Paris, France)
Background/Purpose: Interferon alpha (IFNα) is associated with the severity and disease activity of SLE. Active immunization against IFNα induces polyclonal antibodies against all IFNα subtypes in vivo and prevents severe renal lupus disease in NZW/NZB SLE-prone mice. We evaluate IFNα-Kinoid in a first clinical study in SLE patients.
Method: IFNα-Kinoid (IFNα-K, Neovacs SA, Paris, France) is an immunotherapeutic agent composed of recombinant human IFNα conjugated to KLH as a carrier protein, inactivated and adjuvanted with ISA-51 emulsion.
Twenty-eight patients with mild to moderate, seropositive lupus (SLEDAI 4-10) were enrolled in a double-blind, placebo-controlled, phase 1-2, dose escalation study to evaluate the safety and the immunogenicity of four doses of IFNα-K (30, 60, 120 or 240 mg) administered intramuscularly on Days 0, 7 and 28 with an optional fourth dose on Day 84. Safety evaluation included recording of adverse events and monitoring of haematological and biochemical parameters. Immune responses were measured through titration of anti-IFNα and anti-KLH antibodies, and cellular lymphoproliferation assays. Clinical response was assessed by evaluation of BILAG, SLEDAI, PGA, SRI and titration of serum auto-antibodies, and of IFNα regulated chemokines. PBMC were harvested at several time-points and transcriptomic studies were performed on total RNA using Genechip HGU133 Plus 2.0 arrays.
Results: The safety profile is very favourable. Few minor and transient local and systemic reactions have been observed following immunization. Only minor and transient infections were reported. Two lupus flares were reported as related serious adverse events, one in the active group in a patient who had spontaneously stopped glucocorticoid therapy after the first IFNα-K dose, the other in the placebo group. A dose-related anti-IFNα antibody response was measured in all immunized patients. Antibodies peak after the last dose and decline afterwards.
Transcriptomic studies performed on baseline PBMC samples indicated that the patients cluster into two groups, characterized by the presence or absence of an IFNa signature. Patients with a positive signature have significantly higher dsDNA Ab titers and significantly lower C3 and C4 levels. In the patients with a baseline IFN signature, follow-up transcriptomic studies, performed as early as 38 days after the first IFNα-K injection, showed a significant down-regulation of the expression of IFN-induced genes in the IFNα-K group as compared to the placebo recipients, and, more generally, of SLE over-expressed genes as a whole.
Conclusion: This is the first study to show positive immune and pharmacodynamic results with active immunotherapy against Interferon-α in the treatment of Systemic Lupus Erythematosus. These results are promising and further studies are planned in order to expand upon these observations
Disclosure: F. A. Houssiau, None; R. Rashkov, None; E. Hachulla, None; E. Lazaro, None; C. Jorgensen, None; F. Spertini, None; X. Mariette, None; G. Grouard-Vogel, Neovacs Employee, 3 ; B. Fanget, Neovacs Employee, 3 ; O. Dhellin, Neovacs employee, 3 ; B. Lauwerys, None; P. Vandepapelière, Neovacs employee, 3 .
Source-Newswise