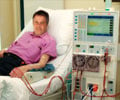
Study says 20 percent of dialysis patients undergoing a percutaneous coronary intervention are given an antithrombotic medication they should not receive
A study in the December 9 issue of JAMA says that some 20 percent of dialysis patients undergoing a percutaneous coronary intervention are given an antithrombotic medication they should not receive, which may increase their risk for in-hospital bleeding.
"In the United States, medication errors are implicated in more than 100,000 deaths annually. Medication errors include adverse drug reactions related to inappropriately prescribed or administered drugs. To minimize inappropriate medication use, the U.S. Food and Drug Administration (FDA) guides pharmaceutical manufacturers and clinicians through drug labeling of which medications are contraindicated or not recommended for use in specific patient groups," the authors write. "Little is known about the use of such medications and their effects on outcomes in clinical practice."Thomas T. Tsai, M.D., M.Sc., of the Denver VA Medical Center and University of Colorado Denver, and colleagues examined the use of the contraindicated/not-recommended antithrombotic agents enoxaparin and eptifibatide among dialysis patients undergoing percutaneous coronary intervention (PCI) and their association with outcomes. The researchers used data from the National Cardiovascular Data Registry (NCDR) from 829 U.S. hospitals on 22,778 dialysis patients who underwent PCI between Jan. 2004 and August 2008. The study focused on the outcomes of in-hospital bleeding and death.
The researchers found that overall, 5,084 patients (22.3 percent) received a contraindicated antithrombotic medication; 2,375 (46.7 percent) received enoxaparin, 3,261 (64.1 percent) received eptifibatide, and 552 (10.9 percent) received both. In unadjusted analysis, patients who received contraindicated antithrombotics experienced higher rates of in-hospital major bleeding (5.6 percent vs. 2.9 percent) and death (6.5 percent vs. 3.9 percent). Further analysis indicated that receipt of contraindicated antithrombotics was significantly associated with increased in-hospital major bleeding, but no significant association was found with in-hospital death.
"This study therefore demonstrates that these medications are used in clinical practice despite FDA-directed labeling, and their use is associated with adverse patient outcomes," the authors write.
"Educational efforts targeting clinicians who prescribe these medications and quality improvement interventions, such as amending clinical pathway order sets to include consideration of renal function, are urgently needed."
Advertisement
RAS