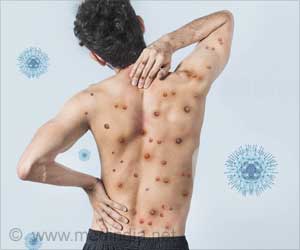
The goal is to develop a commercially useful and effective weapon against bacteria such as methicillin resistant Staphylococcus aureus - better known as MRSA.
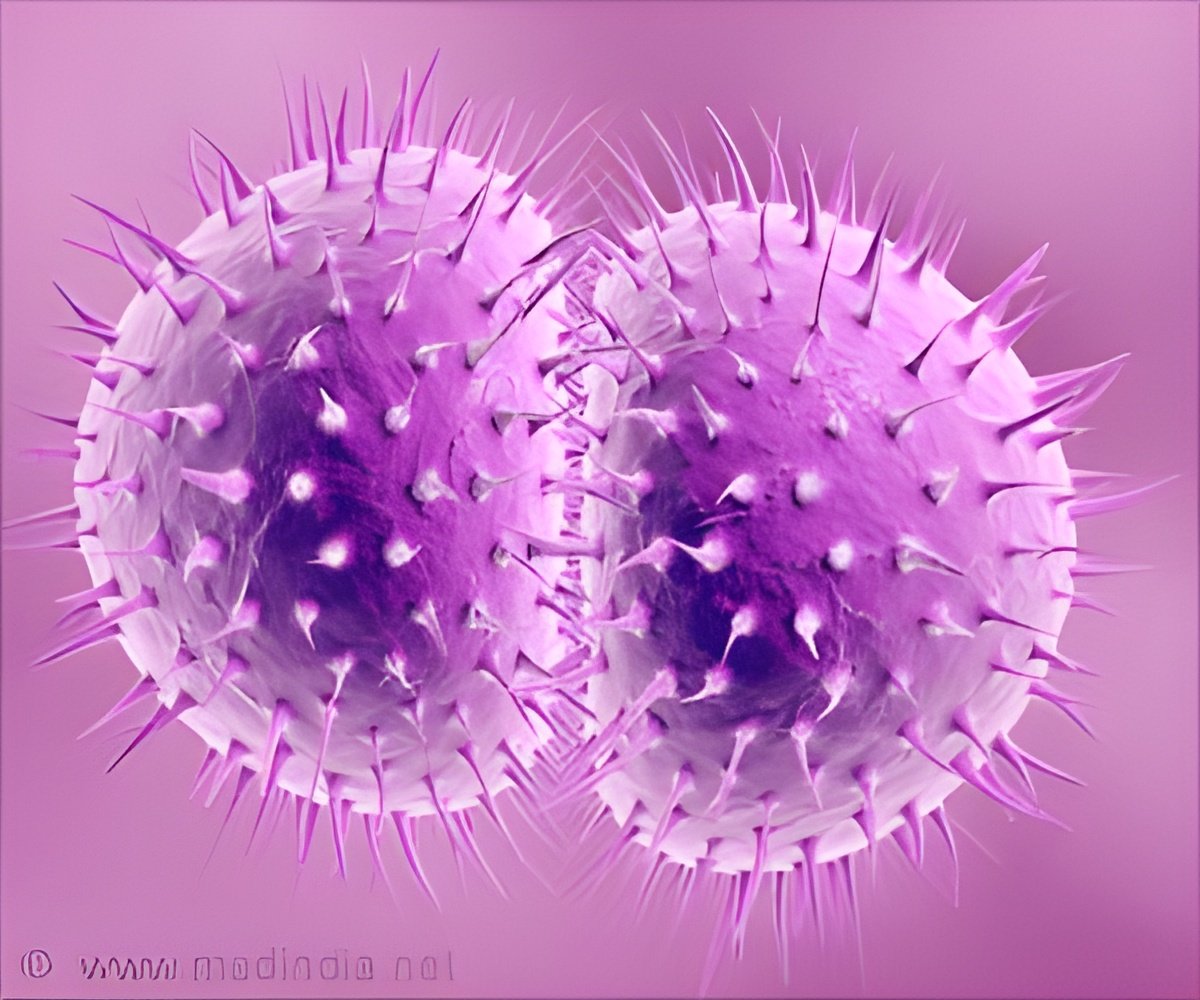
‘Sam Sanderson, Ph.D., a research associate professor in the UNMC College of Pharmacy, recently secured an R01 grant from the National Institutes of Health to find a workable solution’





MRSA is a hardy and potentially deadly strain of bacteria that is notoriously difficult to treat. About 11,285 people die every year as a result of MRSA or MRSA-related infections, according to a 2011 report by the Centers for Disease Control and Prevention. Dr. Sanderson's research will be based on his previous work with EP67, a small protein, or peptide, he and his collaborators created. The synthetic peptide works by stimulating and enhancing a more robust natural immune response to normal and resistant infections, and potentially other ailments such as cancer.
For the NIH study, Dr. Sanderson and his team will focus specifically on tweaking the molecular structure of EP67 into an even more potent tool against MRSA. Once he has identified potentially effective alternate versions of EP67, they will be tested in animals before compiling larger studies to begin the FDA approval process.
"The whole objective of this grant is to lead to an IND (investigational new drug) filing with the FDA and product development," Dr. Sanderson said. "This grant is a great example of genuine translational research that embodies the fusion of academic research and product development with the objective of generating a commercially-available new therapy."
Dr. Sanderson's startup company, Prommune, already has tested EP67's potential against H1N1, and also is looking at its effectiveness against certain parasitic infections.
Advertisement
Earlier this year, Dr. Sanderson teamed with fellow UNMC researcher Joe Vetro, Ph.D., for another R01 grant on a separate project. Funded for $1.75 million, that project will look at EP67-based vaccines against cytomegalovirus or CMV -- a relatively harmless infection in healthy adults, but dangerous to those with a weakened immune system, particularly newborns, infants and the elderly.
Advertisement