Trials with mice were conducted by researchers who injected a specific antibody fragment against soluble aggregates of the A peptide.
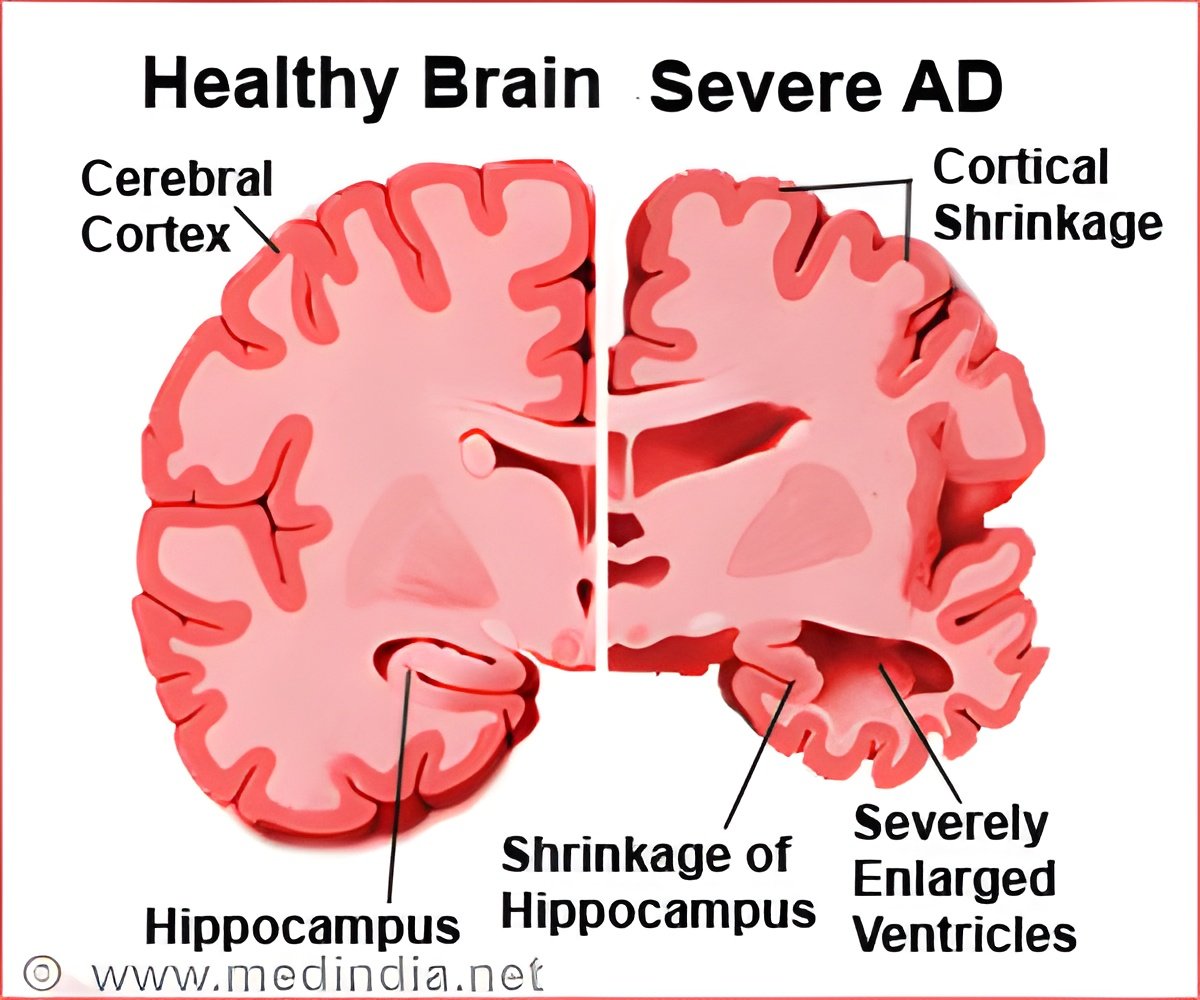
Since the first case of Alzheimer's disease was described, the disease has been associated with the presence of insoluble deposits known as amyloid plaques.
The research group directed by Dr Sandra Villegas, from the Biosciences Unit of the Department of Biochemistry and Molecular Biology at the UAB, designed a recombinant antibody fragment (the single-chain variable fragment scFv-h3D6), a derivative of bapineuzumab, which only consists of the active part trapping the etiological agent of the disease: the domains of the antibody responsible for the binding of Abeta oligomers.
Scientists observed how this antibody fragment protected from cell death in human cell-cultures and described the molecular mechanism by which this antibody fragment removed the Abeta oligomers that cause the disease.
Researchers observed how a single injection into the abdomen of the animals and five days later, the mice reversed their levels of anxiety to normal levels and the learning and memory deficits were ameliorated.
At the molecular level, researchers demonstrated two important facts: first, the treatment cleared from the cerebral cortex the Abeta peptide oligomers, the elements causing the disease; and second, this clearence is linked to the recovery of the levels of certain apolipoproteins suspected to be the natural removers of Abeta peptide aggregates.
Advertisement
Source-ANI













