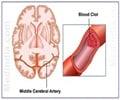
This in-vitro platform permitted quantification and characterization of the pattern and timing of endothelial cell injury with various thrombectomy devices.
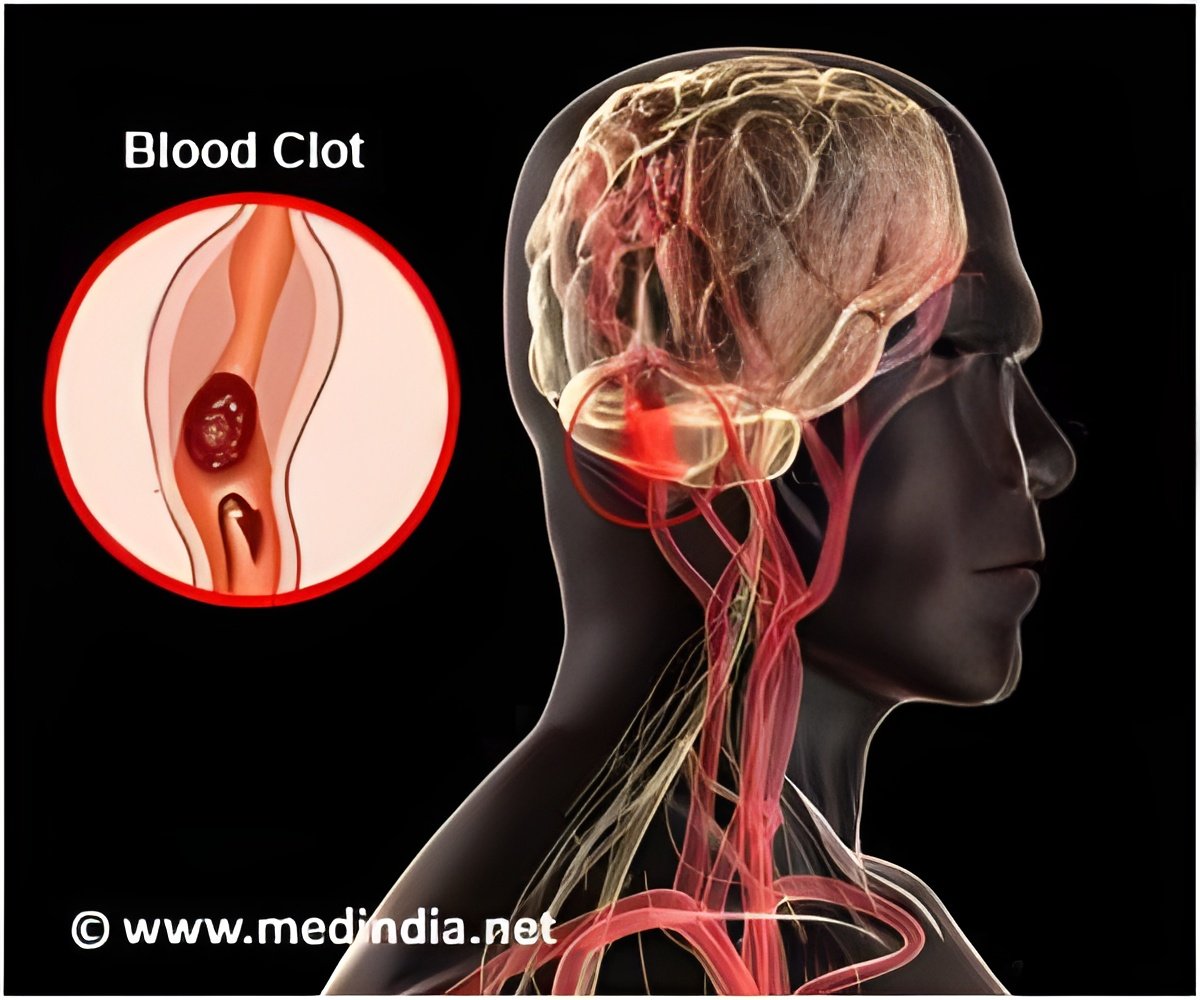
Dr. Alexander Khalessi, director of endovascular neurosurgery and surgical director of neurocritical care at the University of California - San Diego Health System said, "This work offers significant promise going forward. The artificial model could represent a practical, scalable and physiological alternative to existing technologies. For example, in treating cerebrovascular disease that covers a group of dysfunctions related to blood supply to the brain, doctors in certain cases must perform endovascular thrombectomies where they mechanically remove the emboli or clots. The rate of endovascular thrombectomies is rising, but the approach can be improved. Current pre-clinical analyses of any new therapeutic approach or device is limited to either in vitro glass or plastic tube testing intended to mimic biological counterparts or by using animal models, such as pigs. Both of these have significant drawbacks."
The researchers have developed a novel in vitro live-cell platform that allows direct visual characterization of effects and injury patterns to endothelial cells (ECs). Using this they tested various clot-retrieval devices and examined the post-removal effects. Dr. Khalessi said, "We found that the in vitro platform permitted high-resolution quantification and characterization of the pattern and timing of EC injury with various thrombectomy devices and vessel diameters. The devices each displayed different effects." Thereafter, the researchers validated their in vitro findings with in vivo testing.
The findings appear online in Stroke.
Source-Medindia