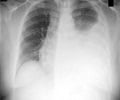
MVA85A, a leading new tuberculosis vaccine has been found to be safe in its Phase I trial.
MVA85A, a leading new tuberculosis vaccine has been found to be safe in its Phase I trial.
Lead researcher Dr. Helen McShane, reader in vaccinology and Wellcome senior fellow at the University of Oxford's Jenner Institute in England, studied the effects of the vaccine specifically in people who had latent tuberculosis infection (LTBI), which can cause full-blown disease when re-activated.For the study, the researchers recruited 12 individuals with LTBI, who did not have other complicating factors like HIV or hepatitis.
"We had two aims in mind with this study," the researchers said.
"First, we wanted to demonstrate that MVA85A was safe in individuals with LTBI, and to ensure we were not inducing any immunopathology with this vaccine. Secondly, we wanted to investigate the immunogenicity of this vaccine in individuals with LTBI and compare that with previous findings in individuals who had been BCG vaccinated," they added.
The patients were vaccinated with the MVA85A vaccine, and followed for 12 months.
Blood tests and diary cards were used to identify any adverse reactions to the vaccine, and the researchers monitored serum inflammatory markers to monitor for any signs of immunopathology.
Advertisement
They also observed that vaccination with MVA85A in LTBI-infected individuals seemed to produce a powerful/potent immune response comparable to previous trials in BCG vaccinated people.
Advertisement
There were no significant increases in serum inflammatory markers.
Dr. McShane says: "The results of this trial are very important, as they suggest MVA85A is safe and highly immunogenic in people who are infected with M. tuberculosis."
She adds: "Further, larger trials are needed in TB endemic areas to assess the efficacy of this vaccine against the development of TB disease, but these results are very encouraging and justify the further development of this vaccine."
The results of the trial have been published in the American Journal of Respiratory and Critical Care Medicine.
Source-ANI
ARU