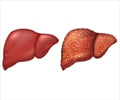
The increasing trend in strategic partnerships between innovator companies, and the US FDA approval for Rezdiffra has led to a boost in the development of medications for MASH.
Novo Nordisk Initiates Groundbreaking Collaborations for Innovative Obesity and MASH Treatments
Go to source).
‘Increase in Strategic Partnerships and US FDA approval of Rezdiffra: A Boon for MASH Patients! #Liverinflammation #MASH #partnerships #medindia’





Advertisement
What is MASH?
MASH was earlier referred to as nonalcoholic steatohepatitis (NASH). It is a type of fatty liver disease marked by liver inflammation and injury due to fat buildup.MASH is frequently linked to conditions such as obesity, type 2 diabetes, and elevated levels of fat in the bloodstream.
Advertisement
Historical Setbacks in the Development of MASH medications
In the past, companies had attempted to develop medications for MASH. However, their studies had to be terminated owing to intense competition and inability to prove efficacy in clinical trials. Some of the potential drugs that had to be discontinued for MASH due to failure in clinical efficacy in their Phase III readouts were Genfit's oral NR1C1/NR1C2 dual agonist Elafibranor, in July 2020, and Bristol Myers Squibb's Pegbelfermin in November 2021.Advertisement
FDA Approval: An Encouragement for the Pharmaceutical Companies
The U.S. Food and Drug Administration (FDA) granted approval to Madrigal Pharmaceuticals’ Rezdiffra (Resmetirom), a THRB small molecule, in March 2024. Rezdiffra is the first to be approved by US FDA for MASH, to be used in conjunction with diet and exercise.The companies are now competing to gain market share in MASH. Further, in recent times, there has been a significant increase in partnerships between drug innovator companies for funding research and development of drugs for the treatment of MASH.
Market Analysis: Global Partnership Deal Value For MASH Drugs
As per GlobalData, a leading data and analytics company, the cumulative worth of collaboration agreements related to MASH innovative medications has risen above $2.5 billion in the year-to-date (YTD) of 2024. It also reported that over $2 billion of this growth was established in the first quarter of 2024.Alison Labya, Business Fundamentals Analyst at GlobalData, states: "Interest in MASH has returned in light of the FDA approval of Madrigal’s Rezdiffra, as well as the success of GLP-1 obesity drugs and their potential efficacy in MASH, as demonstrated by Eli Lilly’s Zepbound (tirzepatide; also known as Mounjaro for type 2 diabetes) in its Phase II SYNERGY-NASH trial readout."
According to the Deals Database of GlobalData's Pharma Intelligence Center, MASH drugs have accomplished a total partnership deal value exceeding $5.7 billion from 2018 to the present year of 2024. It is worth noting that the year 2024 has already emerged as the most profitable year for MASH partnerships in terms of deal value.
Global Partnership Deals
Boehringer Ingelheim entered into a partnership agreement with Suzhou Ruibo Biotechnology, a Chinese biotechnology company, and its subsidiary Ribocure Pharmaceuticals in Sweden in January 2024, with a deal value of more than $2 billion.Boehringer is also proceeding with survodutide, a GLP-1/GLU dual agonist developed in conjunction with Zealand Pharma, which is currently undergoing Phase II trials for MASH.
Labya further mentioned that the partnership between Boehringer and Suzhou Ruibo Biotechnology is the largest collaboration deal in the history of MASH drugs.
In the meantime, Novo Nordisk has signed a co-development partnership worth $500 million with Cellarity, a US-based company, to collaborate on the discovery and development of a small molecule drug for MASH, utilizing Cellarity's artificial intelligence (AI) platform.
Prior to this, Novo Nordisk had also entered into a co-development partnership with Gilead Sciences in March 2021. The purpose of this partnership was to conduct a Phase II study using Novo Nordisk's Ozempic (semaglutide) and Gilead's combination of cilofexor (FXR agonist for liver-related conditions) and firsocostat (an ACC inhibitor). The trial aimed to evaluate the effectiveness of this treatment in patients with MASH and cirrhosis.
Labya further concluded saying: “The recent increase in partnership deals involving innovative MASH drugs in 2024YTD involved large biopharmaceutical companies with prior involvement in MASH drug development, such as Boehringer and Novo Nordisk. Given the large number of MASH drugs in clinical development, including GLP-1 agonists, there may be an increase in partnership deals and mergers and acquisitions involving late-stage clinical MASH drugs over this year.”
In conclusion, the recent approval of Rezdiffra and the strategic collaborations have led to a revival of interest in the development of medications for MASH.
Reference:
- Novo Nordisk Initiates Groundbreaking Collaborations for Innovative Obesity and MASH Treatments - (https://news.europawire.eu/novo-nordisk-initiates-groundbreaking-collaborations-for-innovative-obesity-and-mash-treatments/eu-press-release/2024/01/05/13/06/37/127719/)
Source-Medindia