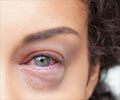
Research conducted by retina surgeons at The Methodist Hospital shows unprecedented improvements in vision in more than one-third of patients treated with Lucentis™ for wet AMD.
Research conducted by retina surgeons at The Methodist Hospital shows unprecedented improvements in vision in more than one-third of patients treated with Lucentis™ for wet age-related macular degeneration (AMD), according to findings published today in the New England Journal of Medicine. AMD is a major cause of central visual loss and is one of the leading causes of blindness in people over 60.
Dr. David M. Brown, retinal surgeon with Vitreoretinal Consultants at The Methodist Hospital, is lead author on the ANCHOR study and a secondary author on the MARINA study. Brown enrolled more patients than any other site worldwide in the studies, which contributed to the U.S. Food and Drug Administration’s June approval of Lucentis for the treatment of patients with wet AMD.“Lucentis therapy is the most significant advance in the treatment of macular degeneration in the history of the disease,” said Brown. “The ANCHOR study compared the previous treatment for macular degeneration (photodynamic therapy) with Lucentis in the most aggressive form of the disease. While 60 percent of the eyes treated with photodynamic therapy were legally blind at the end of the first year of study, patients who received Lucentis treatment were able to avoid legal blindness in seven out of eight cases. Seven percent of the Lucentis-treated patients improved to 20/20 vision at the one year endpoint. It truly made a remarkable difference in many of these patient’s lives enabling them to maintain driving and keep their independence.”
Data from the two randomized, controlled pivotal Phase III clinical trials of Lucentis (ranibizumab injection) shows that at one year, 95 percent of patients treated with Lucentis did not lose vision. Of these patients, 55 percent maintained their vision (defined as a loss of less than 15 letters in visual acuity) and up to 40 percent experienced an improvement of three lines (15 letters) or more on the study eye chart. In the MARINA study, patients were randomized to receive Lucentis injections once a month for two years. Patients in the ANCHOR study were randomized to either receive Lucentis injections once a month or photodynamic therapy every three months for two years.
Lucentis is designed to block new blood vessel growth and leakiness, which lead to wet AMD, by binding to and inhibiting VEGF-A, a protein that is believed to play a critical role in the formation of new blood vessels. Wet AMD affects the macula, the portion of the eye responsible for the central vision required for everyday activities such as reading, driving and recognizing faces. Symptoms include blurred, gray or blank spots in the center of the visual field and distortion that makes edges or lines appear wavy.
The National Eye Institute estimates that there are 1.7 million people with the advanced form of AMD in the United States and that this prevalence will grow to 2.95 million by 2020.
AMD occurs in two forms: dry and wet. While all cases begin as the dry form, wet AMD accounts for about 85 percent of all AMD-related blindness and can result in sudden and severe vision loss. The dry form is associated with atrophic cell death of the central retina or macula. The wet form is caused by growth of abnormal blood vessels that leak fluid and blood under the macula causing scar tissue that destroys the central retina.
Advertisement
SRM