The trail investigates the efficacy and safety of MD1003 drug in the treatment of primary and secondary progressive Multiple Sclerosis.
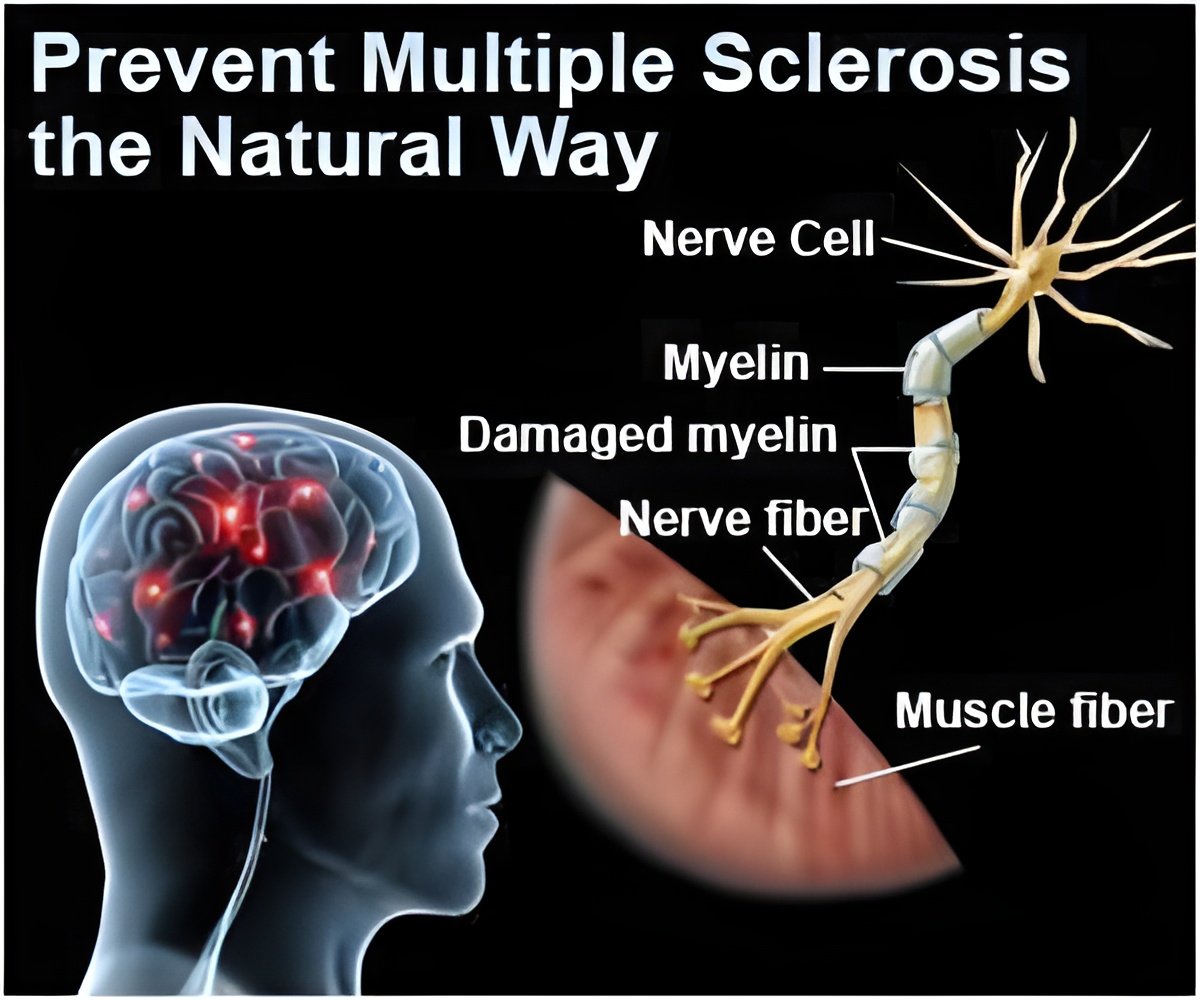
MS-SPI is a randomized, double-blind, multi-center, placebo-controlled trial of MD1003, 300 mg/day, in patients with progressive MS.
A total of 154 patients with a baseline EDSS (Expanded Disability Status Scale) score of between 4.5 and 7 were enrolled from 16 MS reference centers across France. Treatment duration was one year.
Source-Medindia