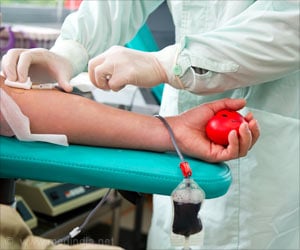
The Food and Drug Administration intends to allow more gay and bisexual individuals to donate blood, which was initially barred due to HIV risk
- A tailored risk assessment is to be introduced by the FDA (Food and Drug Administration) to screen gay and bisexual men before blood donation
- The FDA will most probably support a policy shift to individualized, risk-based donor screening questions in order to reduce the danger of HIV transmission //
- This change in policy can bring inclusivity to the LGBTQ+ community and also reduce the pressure on blood supplies in the country
After significant blood shortages during the COVID-19 pandemic, the duration was reduced to three months. Men who have sex with men would be required to fill out a questionnaire regarding recent sexual activity, among other risk factors, under the proposed plan. The concept, which is still being debated, is to allow those who have not found new partners in the last three months to give.
The Food and Drug Administration said in a statement recently that the new findings "will likely support a policy shift to individual risk-based donor screening questions for minimizing the risk of HIV transmission".
The plan's fundamental elements are contentious among LGBTQ supporters, who claim that previous blood donation policies were discriminatory and that unequal treatment persists.
"I think it is a nominal step in the right direction," said Sarah Kate Ellis, the president and chief executive of GLAAD, an LGBTQ advocacy organization that has been pushing to end the ban for years.
According to an FDA official who requested anonymity to detail internal conversations, the new approach is intended to strike a balance between campaigners who want no restrictions that target gay and bisexual men and blood banks that want to reduce the chance of a recipient contracting HIV.
Several large blood centers are coming to a close on an FDA -funded study aimed at evaluating the safety of replacing the existing three-month waiting period with a more individualized assessment.
The researchers, who enlisted over 1,600 homosexual and bisexual men in eight metropolitan locations, are trying to identify a series of screening questions that can distinguish individuals who have recently acquired HIV from those who have not.
According to Brian Custer, director of Vitalant Research Institute and principal investigator of the study, participants in the ADVANCE study answered a variety of questions about their recent risk-related behaviors, such as whether they had any new sexual partners or were taking pre-exposure prophylactic drugs, known as PrEP, that reduce the risk of HIV infection.
The blood of the subjects was also tested for HIV and the antiretroviral medications used in PrEP.
The researchers have already shared an intermediate analysis with the FDA and expect to have the complete results ready by the end of the year, according to Dr. Custer. He declined to disclose any details about the preliminary findings, citing a confidentiality agreement with the FDA, but said he expected the findings to be useful to the agency.
"I really am confident that we have important information for the FDA to be able to consider what an individual, risk-based approach to donor selection might look like," he said.
Susan Stramer, vice president for scientific affairs for the American Red Cross, said in a statement that the study was meant "to make blood donation a more inclusive process while maintaining the safety of the blood supply".
"While we have not been notified by the FDA concerning policy changes at this time, the Red Cross looks forward to a future in which donation eligibility is not based on sexual orientation and more healthy individuals can give blood to help patients in need," she said.
America's Blood Centers, the trade association that represents 59 community clinics, monitors the current blood shortage on a daily basis. A quarter of the facilities, or 16, had less than a day's supply in hand.
"In this post-pandemic environment, blood drives are hosted by businesses that, in particular, have not returned to pre-pandemic levels as the way people work has changed," Nick Gehrig, the senior director for communications at Vitalant, said in an email.
There has been a constant fight for the LGBTQ+ community to fight for their rights in society worldwide. Just a year ago, the government of India was brought under the radar with a petition that challenged blood donation guidelines, banning transgender persons, members of the gay community, and sex workers from donating blood. With people still dying of the virus, the petition for blood supplies has come under pressure.
Are FDA Restrictions on Blood Donation for Gay and Bisexual Men, Backed by Science?
Infectious diseases such as Hepatitis B, Hepatitis C, and HIV/AIDS are evaluated in donor blood. And a restriction based on the gender identity and sexual orientation projects itself to be arbitrary, illogical, and discriminatory.It's also not scientific, as excluding these populations from donating blood indefinitely and classifying them as high-risk solely on the basis of their gender identity and sexual orientation violates their right to be treated equally with other blood donors.
"It’s not where it should be, though. For years, our community and leading medical experts have said that the FDA's decisions on blood bans for the LGBTQ community are based on stigma, not science, and we’re seeing that pattern continue here," said Sarah.
During a pandemic blood shortage in 2020, the FDA relaxed the regulations, allowing gay and bisexual men who had abstained from intercourse for three months to donate. The rules reinforced the stereotype that these populations were less worthy and submissive in terms of social engagement and healthcare.
Due to the guidelines, many members of the community who needed blood during the epidemic were unable to obtain it from their trans-relatives and loved ones.
Would Gay and Bisexual Men be Willing to Donate Blood if the Guidelines were Changed?
Many gay and bisexual men reported an openness to donating blood if they became eligible; similarly to whole blood donation, they also expressed a strong desire to assist people in need.However, this willingness was exacerbated by the fact that most participants were unfamiliar with plasma donation and unaware of its medical significance.
Participants' views on a policy that allowed this community to donate blood and plasma differed, with some seeing it as a "stepping stone" to a revised blood donation policy and others seeing it as insufficient and gay and bisexual men as "second-class" donors (1✔ ✔Trusted Source
Stepping Stones or Second Class Donors?: a qualitative analysis of gay, bisexual, and queer men's perspectives on plasma donation policy in Canada
Go to source).
Modifying blood and plasma donor policy will require extensive education, an explicit explanation of how this policy change contributes to ongoing or stepwise blood donor policy reform, and significant reconciliation with diverse gay and bisexual men communities, but at the same time, this policy would increase the LQBTQ+ community's inclusivity in society, which is a major concern, and it would also help in reducing the burden of a lack of blood in blood banks.
Reference:
- Stepping Stones or Second Class Donors?: a qualitative analysis of gay, bisexual, and queer men's perspectives on plasma donation policy in Canada - (https://pubmed.ncbi.nlm.nih.gov/33663450/)
Source-Medindia