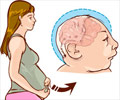
FDA warning has been issued against the use of Paxil in the treatment of depression due to increased risk of congenital heart defects.
A warning has been issued to all physicians, more specifically to pregnant women against the prescription and consumption of Paxil, given for treatment of depression due to the risk of increased congenital heart defects in newborns.
The notice has been issued by the Food and Drug Administration (FDA) and is based on early findings of two studies, which had demonstrated that women who took Paxil during the first trimester had a two fold increased risk of giving rise to babies with congenital heart defects compared to other pregnant women. The risk of birth defect was significantly higher compared to pregnant women who took other forms of anti-depressant medication.In the studies cited by the FDA, the risk of congenital heart defects is about 1 percent overall, which rose to 1.5 to 2 percent in infants born to women taking Paxil. Following the notification, the drug has been placed in the second highest category for risk of birth defects. It is generally advisable to avoid all drugs during pregnancy. However in case use of an anti-depressant medication is very necessary, it is safer to use other medicines as a substitute after a careful consultation with the physician.
The physicians have also been alerted regarding the adverse effects arising out of sudden withdrawal of Paxil. Though it was known that selective serotonin reuptake inhibitors (SSRIs) could harm fetuses, the magnitude of the risk has not been studied previously. The recent report by the FDA and GlaxoSmithKline (manufacturer of Paxil) is the only strong evidence that it can result in birth defects.
Approximately 20% of all pregnant women are estimated to suffer from some form of depression. This creates confusion about the initiation and continuation of anti-depressant medication in pregnancy. The increased tendency of pregnant women to display irritable behaviour, tremors and seizures following childbirth has posed additional concerns regarding mother’s health.
Ever since the time of approval in 1993, the drug has been taken by over millions worldwide. Women of childbearing age (18-45 yrs) constitute 25% of the total number of Paxil users. Although several studies have demonstrated the effectiveness of Paxil in the treatment of depression, anxiety disorder and other psychiatric disorders in adults, the data available on the safe use of Paxil is very limited.
Keeping in mind the above figure, it is high time that certain guidelines are formulated regarding usage of Paxil. Ultimately, the gold standard rule of benefit Vs risk should dictate the indication for use under all circumstances including pregnancy.