During the aging process, a reduction in the levels of protective RNAs may contribute to the onset of Alzheimer's disease.

‘Older individuals with superior memory capacity, known as SuperAgers, were found to have elevated levels of protective short RNA strands in their brain cells. SuperAgers, aged 80 and older, exhibit a memory capacity comparable to individuals 20 to 30 years younger. #alzheimers #RNA’





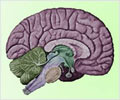
The paper is published in Nature Communications. The Northwestern discovery may have relevance beyond Alzheimer’s. “Our data provide a new explanation for why, in almost all neurodegenerative diseases, affected individuals have decades of symptom free life and then the disease starts to set in gradually as cells lose their protection with age,” Peter said.
The findings also point to a new way for treating Alzheimer’s and potentially other neurodegenerative diseases.
Alzheimer’s is characterized by a progressive occurrence of amyloid-beta plaques, tau neurofibrillary tangles, scarring and ultimate brain cell death.
“The overwhelming investment in Alzheimer’s drug discovery has been focused on two mechanisms: reducing amyloid plaque load in the brain — which is the hallmark of Alzheimer’s diagnosis and 70 to 80% of the effort — and preventing tau phosphorylation or tangles,” Peter said. “However, treatments aimed at reducing amyloid plaques have not yet resulted in an effective treatment that is well tolerated.
Advertisement
Such drugs exist, Peter said, but they would need to be tested in animal models and improved.
Advertisement
Difference between Toxic and Protective Short RNAs
All our gene information is stored in form of DNA in the nucleus of every cell. To turn this gene information into the building blocks of life, DNA needs to be converted into RNA which is used by cell machinery to produce proteins. RNA is essential for most biological functions.In addition to these long coding RNAs, there are large numbers of short RNAs (sRNAs), which do not code for proteins. They have other critical functions in the cell. One class of such sRNAs suppresses long coding RNAs through a process called RNA interference that results in the silencing of the proteins that the long RNAs code for.
Peter and colleagues have now identified very short sequences present in some of these sRNAs that when present can kill cells by blocking production of proteins required for cells to survive resulting in cell death. Their data suggest that these toxic sRNAs are involved in the death of neurons which contributes to the development of Alzheimer’s disease.
The toxic sRNAs are normally inhibited by protective sRNAs. One type of sRNA is called microRNAs. While microRNAs play multiple important regulatory roles in cells, they are also the main species of protective sRNAs. They are the equivalent of guards that prevent the toxic sRNAs from entering the cellular machinery that executes RNA interference. But the guards’ numbers decrease with aging, thus allowing the toxic sRNAs to damage the cells. The amount of protective sRNAs is reduced in the aging brain. Adding back protective miRNAs partially protects brain cells engineered to produce less protective sRNAs from cell death induced by amyloid beta fragments (which trigger Alzheimer’s). Enhancing the activity of the protein that increases the amount of protective microRNAs partially inhibits cell death of brain cells induced by amyloid beta fragments and completely blocks DNA damage (also seen in Alzheimer’s patients.)
Scientists analyzed the brains of Alzheimer's disease mouse models, the brains of young and old mice, induced pluripotent stem cell-derived neurons from normal individuals (both young and aged) and from Alzheimer's patients, the brains of a group of older individuals over 80 with memory capacity equivalent to individuals 50 to 60 years old, and multiple human brain-derived neuron-like cell lines treated with amyloid beta fragments, a trigger of Alzheimer’s.
Reference:
- Death Induced by Survival gene Elimination (DISE) correlates with neurotoxicity in Alzheimer’s disease and aging - (https://www.nature.com/articles/s41467-023-44465-8)