U.S. scientists have discovered a novel tool for distinguishing among root causes of iron overload or deficiency in humans.
A novel tool for distinguishing among root causes of iron overload or deficiency in humans has been discovered by US scientists.
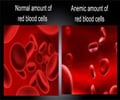
Reporting their work in the journal Cell Metabolism, University of Utah researchers in Salt Lake City highlighted the fact that iron helps the body produce haemoglobin that enables red blood cells to carry oxygen, but too much iron could bind up and eventually damage organs.The researchers said that the balance of iron in mammals is controlled by a liver-produced hormone called hepcidin and the iron transporting receptor ferroportin.
Hepcidin binds ferroportin to stimulate its break down, thereby lowering iron export. While too much hepcidin results in anaemia, too little of it disables the body to get rid of enough iron.
Now, the researchers have identified the critical hepcidin-binding domain (HBD) on ferroportin.
According to them, by placing that binding site on a bead, they now have a very specific method for detecting hepcidin levels in human blood.
"We've identified the hepcidin-binding site. It will allow the diagnosis of underlying inflammation to distinguish diseases of iron metabolism that stem from hepcidin versus those with other causes," said Jerry Kaplan, a university researcher.
Advertisement
He further said that given that microorganisms need iron, increases in hepcidin that lead to a decline in ferroportin and iron were believed to be antimicrobial.
Advertisement
They said that while other tests for hepicidin have been developed, the new assay was unique in that it specifically identifies the hormone's biologically active form.
They believe that the new assay could also be used in other vertebrates due to remarkable degree of evolutionary conservation of the binding site.
"This test narrows it down to (active hepcidin). It can help us divine the effects of inflammation on body iron stores," Ward added.
Apart from that, the researchers have also discovered that human hepcidin binds ferroportin at 37 degree Celcius, but not at four degree Celcius.
It so happens because the hepcidin from humans changes its conformation at low temperatures, they say.
Source-ANI
RAS/L