An infinite number of melodies, harmonies and rhythms can be produced by a small ensemble of musicians.
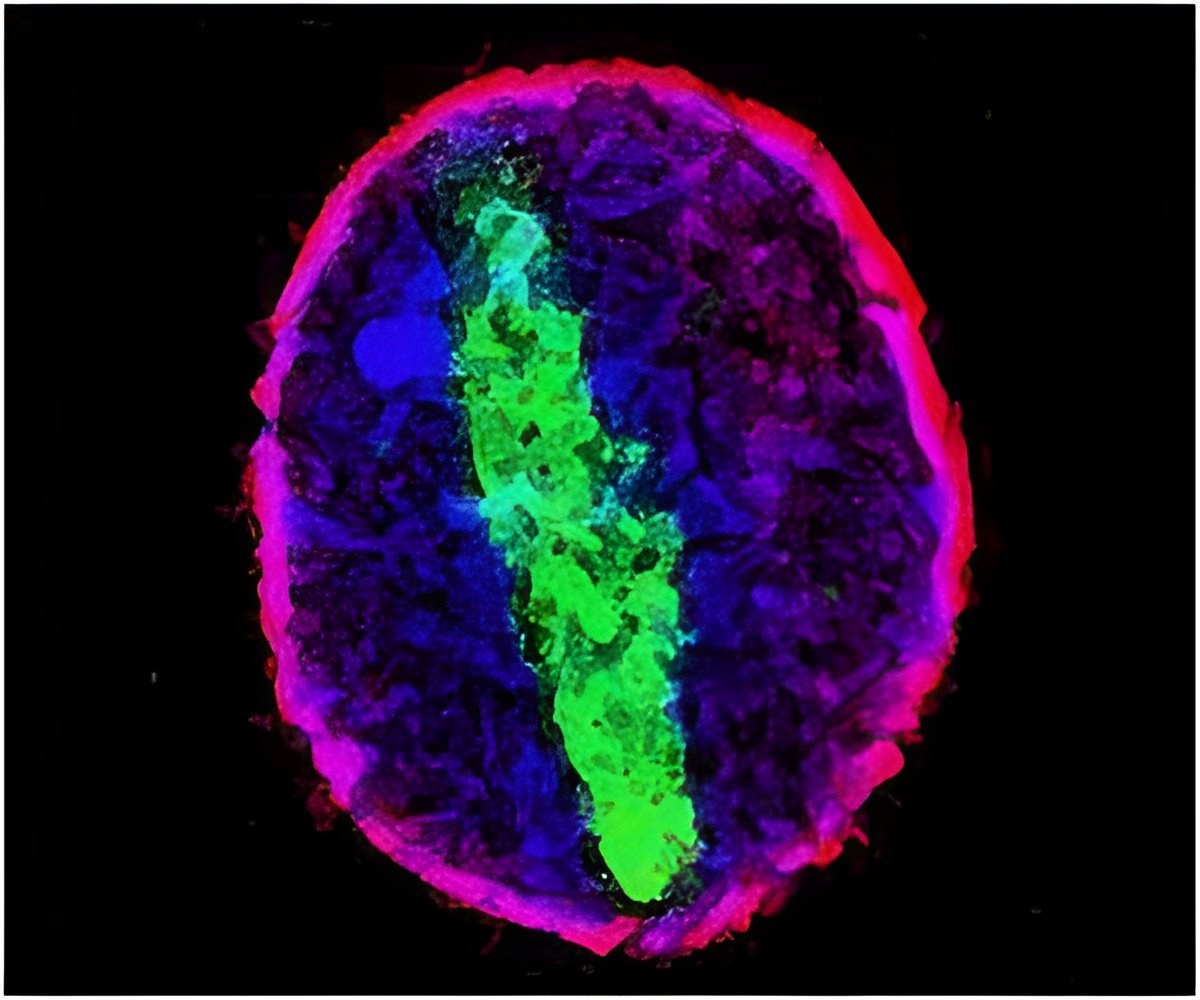
A new study by Stowers Institute for Medical Research Investigator Ting Xie, Ph.D., reveals yet another duet played by Notch and BMP signals, this time with Notch calling the tune. That work, published in this week's online issue of PNAS, uses mouse genetics to demonstrate how one Notch family protein, Notch2, shapes an eye structure known as the ciliary body (CB), most likely by ensuring that BMP signals remain loud and clear.
In vertebrates, the CB encircles the lens and performs two tasks essential for normal vision. First, it contains a tiny muscle that reshapes the lens when you change focus, or "accommodate". And it also secretes liquid aqueous humor into the front compartment of the eye where it likely maintains correct eye pressure. Understanding CB construction is critical, as excessive pressure is one risk factor for glaucoma.
Eye development is a relatively new field for Xie, a recognized leader in the study of adult stem cells in the fruit fly: only recently did he branch out into mouse studies. "A few years ago I was asked to participate in a think tank-type meeting to discuss the potential application of cell therapy to treat glaucoma," he says. "I became interested in using retinal progenitor cells to treat diseases like glaucoma or macular degeneration. But I realized that first we needed to understand eye disease at the molecular level." The new study is an important step in that direction.
Previously, investigators knew that once cells that form the CB are established in an embryo, the BMP pathway drives their "morphogenesis", the term used by developmental biologists to describe the process of expanding and then sculpting a committed population of cells into a unique structure. "The Notch2 receptor was previously shown to be expressed in the developing mouse eye," explains Chris Tanzie, M.D., Ph.D., a former graduate student in the Xie lab and the study's co-first author. "But its function was unknown, and no one connected how various signaling pathways direct CB morphogenesis."
To determine what Notch2 was doing in the developing eye, the Stowers team constructed a conditional knockout mouse, meaning that the Notch2 gene is deleted from the genome only in eye cells that give rise to the CB. In normal newborn mice a series of cellular "folds" that characterize the CB emerges over the first 7 days of life. But the mutant knockout mice showed a complete absence of folds, dramatic evidence that Notch2 is required to elaborate a CB.
Advertisement
Biochemical and microarray analysis provided further explanation for defects observed after Notch2 loss. Comparison of normal and Notch2-mutant eye cells revealed that not only did cells of mutant mice lose BMP signaling but that expression of two proteins known to interfere with BMP increased in those cells.
Advertisement
The study's second co-first author is Yi Zhou, a University of Kansas Medical Center graduate student earning his Ph.D. in Xie's lab. "Our work reveals a novel link between Notch and BMP pathways potentially involved in the pathogenesis of glaucoma," says Zhou, noting one more tantalizing implication of the paper. "In addition, mutations in Jagged-1 and Notch2 are thought to underlie the human genetic disease known as Alagille Syndrome. Our work may lead to a better understanding of both."
Alagille Syndrome is an inherited childhood disorder causing defects in organ systems including liver, heart and the skeleton. Xie is equally intrigued by potential connections between his group's observations in the mouse eye and Alagille outcomes in humans. Nonetheless, he remains focused on nailing down how perturbation of the Jagged1-Notch2-BMP axis might cause eye disease.
"We now know how to build better mouse mutants to study CB development. In this work we show that Notch regulates BMP signaling but have not yet determined whether alterations in CB structure actually change interocular pressure," he says. "Answering that question is our future goal."
Source-Eurekalert