US health officials said Wednesday the Massachusetts compounding pharmacy that sold a steroid preparation linked to a deadly meningitis outbreak had significant in-house hygiene issues.
She said that records showed that NECC did not sterilize products for the minimum time required to ensure sterilization, and failed to properly maintain sterilization equipment.
Worse, her office found that on 13 occasions, NECC staff shipped orders from the suspect medication lots before receiving test results confirming that the lots were sterile.
"Medication was shipped as long as 11 days before the results were received," Biondolillo said.
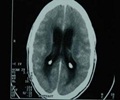
The unprecedented outbreak of fungal meningitis linked to the contaminated drug has killed 23 people.
The number of infections tied to the tainted steroid rose to 308 in 17 states, the Centers for Disease Control and Prevention reported on its website Tuesday.
Advertisement
The southern state of Tennessee remains the hardest hit with 70 cases and nine deaths, followed by Michigan with 68 cases and five fatalities.
Advertisement
The tainted steroid -- typically injected into the spine to treat back pain -- was produced by NECC -- which has since shut down its operations and recalled all of its product.
Source-AFP