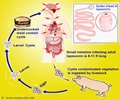
Critical insight into the biochemistry of a rare and fatal form of epilepsy known as Lafora disease, a genetic condition that typically strikes children in their teens is offered in a new study
That enzyme, known as glycogen synthase, usually tacks on glucose molecules, but every so often it will incorporate a stray phosphate molecule instead. Most of us depend on another enzyme called laforin to fix those errors. In people with Lafora disease, many of whom carry a mutated version of laforin, those phosphate molecules are allowed to accumulate, producing what Peter Roach of Indiana University School of Medicine refers to as "glycogen gone wrong" and the formation of deposits called Lafora bodies in many organs and, most devastatingly, in neurons.
"We view this as a catalytic error," Roach said. "The mistake is damaging enough that a mechanism is in place to get rid of it." Roach compares this metabolic correction mechanism to the repair processes that have evolved to correct errors in DNA synthesis.
Glycogen molecules can be extremely large and those molecules are constantly degraded and rebuilt, he explained. As a result, phosphate levels can build until they affect the overall chemical properties of the glycogen. That explains why symptoms of the disease take time to appear. Symptoms of Lafora disease typically set in during the teenage years. The epileptic condition and its neurological symptoms then grow progressively worse, usually leading to death in about 10 years time.
Earlier studies had shown that glycogen phosphate could be released by laforin and that excessive phosphate accumulation in glycogen affected the structure and properties of glycogen. Roach and his colleagues set out to work out the additional molecular details.
Their first question was how the phosphate gets into glycogen in the first place. They suspected there might be a special enzyme responsible, but instead found that glycogen synthase is the source of the problem. That enzyme mistakenly incorporates phosphate at a rate of about one phosphate per 10,000 glucoses. Roach's team also found exactly where it is on the glycogen molecule that those phosphates land, a feat that he says was rather difficult to do given that phosphate is such a rare constituent in the molecule.
Advertisement
It might be possible to limit the activity of glycogen synthase specifically in the brain, he continued. While one would want to be careful not to disrupt that enzyme in other parts of the body, earlier studies have shown that mice lacking glycogen in their brains altogether appear to function normally.
Advertisement
As for the future, Roach said that there is a second gene that can also lead to Lafora disease and his group intends work out the underlying mechanisms there as well.
Source-Eurekalert