The first preliminary steps toward developing a form of gene therapy for sickle cell disease has been initiated by researchers at Dana-Farber/Children's Hospital Cancer Center (DF/CHCC).
"This work builds on the transformative basic research discovery of the role of BCL11A in maintaining fetal hemoglobin silencing by the Orkin laboratory with a near-term goal of curing sickle cell disease using gene therapy," said Williams, chief of the Division of Hematology/Oncology at DF/CHCC and senior author on the abstract. "We have had important recent successes in applying this type of gene therapy in treating several other genetic diseases at Boston Children's Hospital."
The team's work builds on previous research, published by Orkin's laboratory, suggesting that strategies targeting a molecular switch called BCL11A have the potential to correct sickle cell disease.
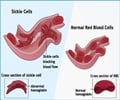
First described over 100 years ago, sickle cell disease (or sickle cell anemia) is an inherited blood disease caused by a single mutation in one of the components of hemoglobin, the oxygen-carrying protein in red blood cells. The mutation reduces the protein's ability to carry oxygen and forces the cells to curve into a distinctive crescent or sickle shape.
Our bodies can actually manufacture two forms of hemoglobin: the adult form susceptible to the sickle cell mutation, and a fetal form that is largely produced during development and for a short time after birth. BCL11A is a transcription factor that facilitates that shift in hemoglobin production. Fetal hemoglobin expression significantly reduces the symptoms and complications of sickle cell disease because it prevents the intracellular abnormalities caused by the sickle mutation.
In their abstract, Renella, Orkin, Williams and their colleagues reported on their efforts to develop a combined lentiviral gene transfer/RNA interference approach capable of turning down BCL11A in vitro in human and mouse cells and in vivo in a mouse bone-marrow transplant model. Among their findings, the researchers documented a five- to 20-fold increase in fetal hemoglobin production in treated mice.
Advertisement
Advertisement
Source-Eurekalert