In a study of dark-skinned mice, Stanford researchers have found that a common protein plays a vital role in tumor suppression, a finding that may ultimately lead to new treatments
Stanford researchers have found that a common protein plays a vital role in tumor suppression. This study in mice may lead to new treatments for bone marrow failure in humans.
The study at the Stanford University School of Medicine has dubbed this protein, called p53, as the "guardian of the genome" for its ability to recognize DNA damage and cut short the division of potentially cancerous cells.But, on the other hand, it seems that p53 also responds to disruptions in the cell's protein factories, leading to changes in skin colour and causing anaemia in mice.
"This may be just the tip of an iceberg. When we think of p53, we think in extremes: high levels cause cell death, low levels cause cancer. This research shows that even moderate changes can have very important consequences. It also suggests that the activation of p53 may be involved in more pathways than we previously anticipated," The Nature quoted said Gregory Barsh, MD, PhD, professor of genetics and of paediatrics.
While studying the mutations that darken the feet, tails and ears of normally light-skinned mice, the researchers closed in on two skin-darkening mutations, which were found to affect specific protein components of the cell's ribosomes.
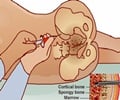
This discovery holds importance as mutations affecting one of the same ribosomal proteins in humans are linked with Diamond-Blackfan syndrome, a condition that causes a type of anemia specific to red blood cells. And after analysing the dark-skinned mice more closely, it was found that these mice showed similar abnormalities in red blood cell formation.
"Diamond-Blackfan itself is fairly rare, but the bone marrow failure that sometimes occurs in these individuals happens quite often in many other disorders, including acute myelogenous leukaemia and multiple myeloma," said McGowan.
Advertisement
As people with mutations in the same ribosomal protein exhibit varied symptoms, it led the scientists to suggest that although the mutations occur in the all-important ribosomes, the problem isn't the result of ham-handedly interfering with all protein production in the cell.
Advertisement
On the contrary, mutant mice unable to express p53 were found to have normal levels of Kit ligand. They also had light-coloured feet and unaffected numbers of red blood cells.
"The involvement of p53 in this pathway suggests that the variability seen in human disease may be due to a varying extent to which p53 is activated, or expressed. The mild anemia seen in these mice and in some humans with Diamond-Blackfan syndrome may be due to mild activation of p53. More severe anemia or bone marrow failure may be the result of very high levels of p53 activation," said McGowan.
The researchers speculated that increased activation of p53 affects different types of cells in the body in differently. In skin cells, it increases the amount of Kit ligand and causes darker skin, and in bone marrow cells it causes anaemia by causing the death of red blood cell precursors.
All these findings suggest that moderating the levels of p53 can be a possible way to treat a variety of bone marrow failures in humans.
The researchers now plan to develop a better mouse model of bone marrow failure to try new drugs and therapies. They will also search for additional skin-darkening mutations that affect this and other previously unknown p53 pathways.
"This illustrates the potential benefits that come from basic science research. Although you don't always know where you're going to end up, many advances in human health would not have been discovered any other way," said Barsh.
The study was published online in the recent issue of Nature Genetics.
Source-ANI
RAS/L