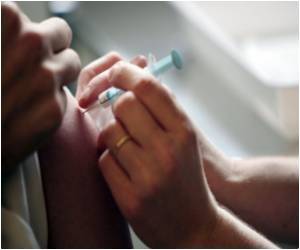
Though associated with a reduction in both herpes zoster and postherpetic neuralgia, the uptake of shingles vaccine in the US is low.
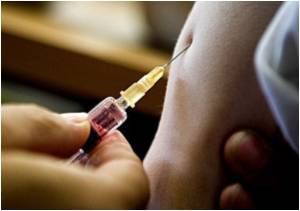
The researchers, led by Sinéad Langan from the London School of Hygiene and Tropical Medicine, reached these conclusions by examining the records of 766,330 Medicare beneficiaries aged 65 years or more between 2007 and 2009.
They found that shingles vaccine uptake was extremely low—only 3.9% of participants were vaccinated—but was particularly low among black people (0.3%) and among people with a low income (0.6%).
Over the study period, almost 13,000 participants developed shingles and the vaccine reduced the rate of shingles by 48% (that is, approximately half as many vaccinated individuals developed shingles as those who were not vaccinated). However, the vaccine was less effective in older adults with impaired immune systems. The authors also found that vaccine effectiveness against post-herpetic neuralgia was 59%.
The authors say: "Herpes zoster vaccination was associated with a significant reduction in incident herpes zoster and [post-herpetic neuralgia] in routine clinical use."
They continue: "Despite strong evidence supporting its effectiveness, clinical use remains disappointingly low with particularly low vaccination rates in particular patient groups."
Advertisement
Advertisement