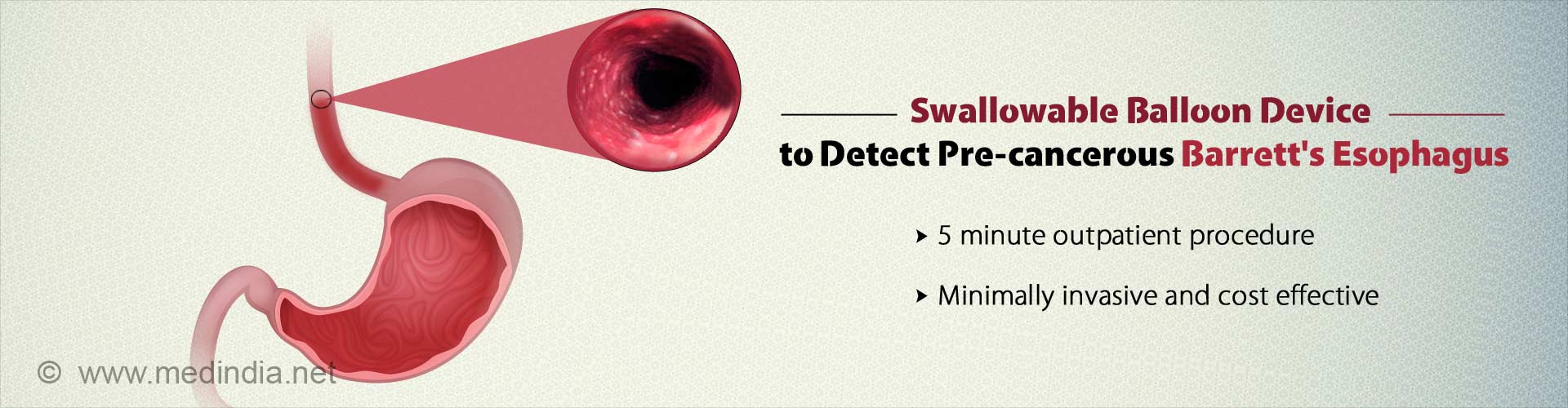
Rapid, minimally invasive and cost-effective swallowable balloon device , can now be used to identify cancer of the esophagus.
- New swallowable balloon device detects pre-cancerous Barrett's esophagus in a fast, minimally invasive and cost effective way.
- Barrett's esophagus (BE) is the precursor lesion of esophageal adenocarcinoma, which if detected earlier can prevent the lethal cancer.
- The five minute outpatient test which is more than 90% sensitive for detecting BE could be a better alternative than the traditionally used endoscopy.
Barrett's Esophagus and its Diagnosis
Barrett's esophagus (BE) is the precursor lesion of a highly lethal cancer called esophageal adenocarcinoma (EAC). EAC can be prevented if patients are diagnosed at the precursor stage of Barrett's esophagus. However, traditional BE detection methods include endoscopy, which is an expensive and invasive screening test that requires sedation and hence is not preferred for routine screening. "Our goal is early detection," said Dr. Amitabh Chak, Professor of Medicine and head of the NIH-Case Barrett's Esophagus Translational Research Network "BETRNet" program "Symptoms of Barrett's esophagus, such as heartburn, can also be commonly seen in individuals who have acid reflux disease without BE. These symptoms can easily be treated by over the counter medications so people often don't get tested for BE, particularly by an invasive test such as endoscopy. As a result, when individuals develop EAC, 95 percent of the time the presence of the prior Barrett's esophagus was undetected and unknown. We wanted an easier, less costly test that could provide a practical way for screening and early detection of individuals with BE, who can then be followed closely to prevent development of EAC."The Swallowable Test
The new test is an easy, five minute outpatient procedure which is more than 90 percent sensitive for detecting individuals with BE. Patients are simply required to swallow a pill sized balloon that swabs the esophagus. The pill once retrieved through the mouth, is tested for DNA mutations that the team has discovered are diagnostic of BE.Paralelly, Helen Moinova, PhD, the first author of the study led another study to identify DNA changes diagnostic of Barrett's. Using genome-wide sequencing approaches, Dr. Moinova identified two genes, VIM and CCNA1, that get chemically modified by DNA methylation in BE. The DNA assay showed 90.3% sensitivity for detecting individuals with BE and 91.7% specificity for correctly identifying individuals who were normal.
"Having two accurate biomarkers increases confidence in our ability to correctly diagnose Barrett's esophagus," said Dr. Moinova. "Taken together, our findings show that non-endoscopic balloon sampling paired with molecular tests for the methylated VIM and CCNA1 biomarkers is effective in addressing the need for simple, non-invasive, safe, and accurate Barrett's esophagus screening."
The Clinical Trial
The clinical trial included 86 individuals who were tested for BE using the swallowable esophageal balloon device.The participants were asked to swallow the device, which was attached to a thin silicone catheter. Once the pill was delivered to the stomach, the small balloon was inflated by injecting air through the catheter. The inflated balloon was maneuvered to swab the lower esophagus near the stomach, the place where BE begins, and obtain a sample of the lining cells. After collecting the sample, the balloon was deflated and inverted back into the capsule, to protect the sample. The capsule was then retrieved through the mouth and DNA was extracted from the balloon to perform Dr. Moinova's DNA assay.
The procedure was well tolerated by the patients. 82 percent reported little to no anxiety, pain, or choking. 93 percent stated they would repeat the procedure again. 95 percent said they would recommend the test to others.
The positive results from the clinical trial show that the new screening method can significantly change the method and increase the efficacy of BE screening.
Reference:
- H.R. Moinova el al., "Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett's esophagus," Science Translational Medicine (2018).DOI: 10.1126/scitranslmed.aao5848
Source-Medindia