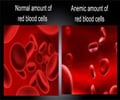
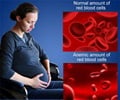
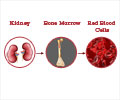
Using a small interfering RNA (siRNA) drug to stimulate natural EPO production by the liver can help avoid the risks.
Marc T. Abrams and colleagues, Merck Research Laboratories (West Point, PA and Boston, MA), designed a siRNA drug that targets and inhibits expression of the EGLN1 gene, thereby blocking production of a protein called prolyl-4-hydrolase 2 (PHD2). The liver normally makes only small amounts of EPO in adult primates and humans, but one dose of the siRNA drug led to increased levels of EPO and hemoglobin in the blood of the primates. The siRNA effect was dose-dependent and was sustained for at least two months, report the authors in the article "A Single Dose of EGLN1 siRNA Yields Increased Erythropoiesis in Nonhuman Primates."
"The translational relevance of this paper is that it successfully advances the in vivo therapeutic investigation of PHD inhibitors from previous mouse-based work to achieve increased serum EPO and hemoglobin in a primate model, " says Executive Editor Graham C. Parker, PhD, The Carman and Ann Adams Department of Pediatrics, Wayne State University School of Medicine, Children's Hospital of Michigan, Detroit, MI.
Source-Eurekalert