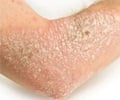
Pain-sensing nerves also help fight skin infections and prevent its spread, reveals a new study published in Cell. Researchers suggest this new type of immunity as anticipatory immunity .
‘The study finding would help establish specific therapeutic approaches for autoimmune diseases of barrier tissues (skin, gut), like psoriasis.’





Until about a decade ago, pain was thought to have evolved as a way for your body to tell you to stay away from a particular stimulus or to signal a problem with its function, like an injury. More recently however, researchers have shown that it may play an important role in immunity against some pathogens. In the study, Kaplan and first author Jonathan Cohen, an M.D./Ph.D. student in Kaplan’s lab, collaborated with Pitt neurobiology professors and pain experts Kathy Albers, Ph.D., and Brian Davis, Ph.D., to develop an optogenetic mouse model where pain sensing neurons in the skin could be activated by shining blue light.
They first showed that just activating these neurons released a small protein called CGRP, which recruited different types of immune cells to the site. This suggested that neurons detecting skin pathogens on their own kickstart an immune response even before sentry immune cells could.
Then in the same mouse model, they infected the animals with either Candida albicans, a fungus that causes candidiasis, commonly known as thrush, or Staphylococcs aureus, a common bacterium that can turn deadly under certain conditions.
Using optogenetics and chemical nerve blockers, the researchers showed through a series of elegant experiments that when the fungus infected the skin at one location, the nerves not only detected and initiated an immune response to fight the infection, but also sent a signal toward the spinal cord. Those signals then boomeranged back to skin at areas around the infection to activate immune defenses in anticipation, thereby preventing the infection from spreading.
Advertisement
"The advantage of involving the nervous system is that it can communicate information across a space in a span of milliseconds, compared to hours or days for the immune cells to do the same function," said Jonathan Cohen, an M.D./Ph.D. student in Kaplan’s lab and the first author of the study. "It’s the difference between sending Paul Revere to warn of the British advance and sending a telegram to do the same."
"Understanding this really new type of immunity raises the intriguing question of whether we could develop a drug to selectively suppress excessive autoimmune inflammation in specific tissues, avoiding the negative side effects that come with using a broad immunosuppressant that affects the entire body," he says.
Source-Eurekalert