One of the leading causes of vision loss is a condition called neovascular age-related macular degeneration.
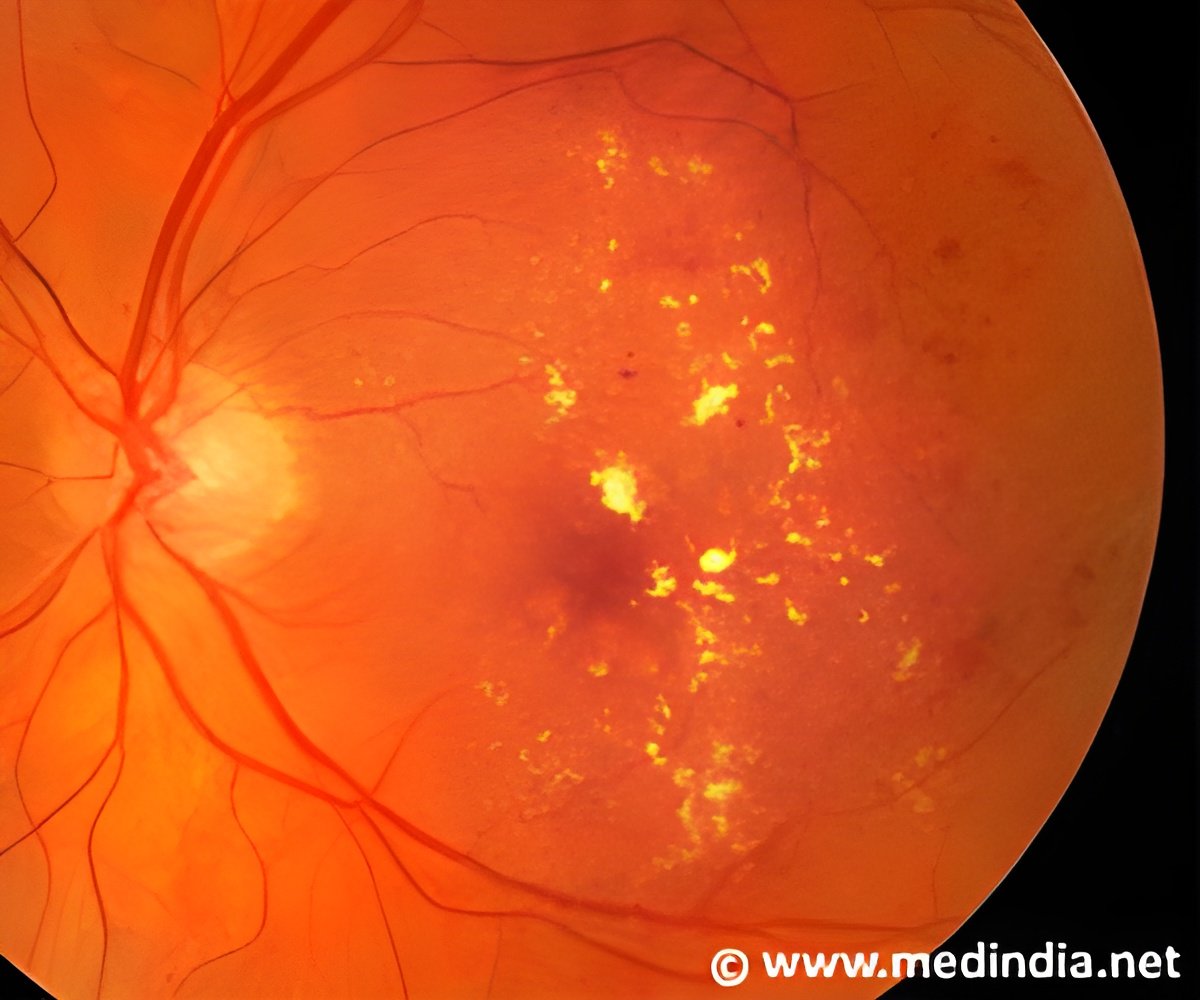
Their hope is this new model will help them understand AMD and develop new treatments for the disease. The new mouse strain was generated through the Jackson Laboratory Eye Mutant Screening Program. The Jackson Laboratory is an independent, nonprofit organization focusing on mammalian genetics research to advance human health. The investigation, led by Kip Connor, Ph.D., HMS Assistant Professor of Ophthalmology and Assistant Scientist at Mass. Eye and Ear , demonstrated that NRV2 mice show multiple areas of retinal depigmentation and neovascularization, which occur spontaneously and are concurrent with the presence of the abnormal blood vessels in the retina during early postnatal days.
Furthermore, they showed that the NRV2 neovascularization originates from the retinal vascular plexus and grows toward the subretinal space. Neovascular features in the NRV2 mouse strain mimic the early clinical stages of RAP in humans. "It is our hope that future studies using this mouse model will allow us to develop novel, specific therapeutics that result in better visual outcomes and quality of life for patients suffering from AMD, including RAP," Dr. Connor explained.1In the study, researchers focused on the characterization of the ocular phenotype and etiology of angiogenesis in NRV2 mice. The phenotypic changes within the retina in NRV2 mice were evaluated by fundus photography, fluorescein angiography, optical coherence tomography, immunohistochemical and electron microscopic analysis.
The pathological neovascularization was imaged by confocal microscopy and reconstructed using three-dimensional image analysis software. The researchers found that NRV2 mice developed multifocal retinal depigmentation in the posterior fundus spontaneously. The vascular leakage observed correlated with the areas of depigmentation by fluorescein angiography. Moreover, the three-dimensional reconstruction of retinal vasculature clearly revealed that the spontaneous angiogenesis arose from the retinal vascular plexus at postnatal day (p)15 and extended through the outer retina toward the subretinal space. "Despite several mouse models of retinal angiogenesis, the underlying etiopathogenesis and mode of disease progression in AMD patients with RAP remain to be elucidated," said Eiichi Hasegawa, Ph.D., lead author of the paper. "We anticipate this mouse model could be a useful tool to define the molecular pathway and the specific genes involved in retinal angiogenesis and development of novel anti-angiogenic therapies."
Source-Eurekalert