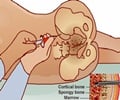
A new study suggests that the current stem cell transplant regimen to treat advanced neuroblastoma in children in the United States is more toxic.

Both approaches to high-risk neuroblastoma employ high doses of chemotherapy to eradicate cancer cells followed by infusion of the patient's previously collected stem cells to allow the patient to recover more quickly and safely. Since 2007, physicians at DF/CHCC and others in the Children's Oncology Group had been using a combination of high-dose carboplatin, etoposide, and melphalan to prepare patients for transplant, said Lehmann. European centers have preferred busulfan and melphalan over the platinum-based regimen.
"We have had a long-standing collaboration with Children's Cancer Hospital Egypt in Cairo, which is under the leadership of senior author Dr. Alaa Elhaddad," Lehmann said. "We decided to compare the toxicities in patients who received care that was very similar except for the drugs used in the preparative regimen."
"We found there was no difference in survival, but our regimen was associated with more liver and kidney toxicity and more bloodstream infections," she noted. "This was very useful information as COG contemplated switching to the European approach."
In addition to the idea of using toxicity data to choose between approaches of similar efficacy, Lehmann noted, "This study demonstrates you can have true collaboration between transplant centers located in very different parts of the world."
First author of the study is Yasser Elborai, MD, of the Children's Cancer Hospital Egypt.
Advertisement
--Written by Richard Saltus, Dana-Farber/Children's Hospital Cancer Center.
Advertisement
Since 1947, Boston Children's Hospital and Dana-Farber Cancer Institute have provided comprehensive care for children and adolescents with cancer through Dana-Farber/Children's Hospital Cancer Center. The two Harvard Medical School affiliates share a clinical staff that delivers inpatient care at Boston Children's and outpatient therapies at Dana-Farber's Jimmy Fund Clinic. The Boston Children's inpatient pediatric cancer service has 33 beds, including 13 designated for stem cell transplant patients.
Boston Children's is also the site of DF/CHCC inpatient clinical translational research in pediatric malignancies and has long supported the operation of an effective and productive stem cell transplant service. It has a long history of investment in and support of both clinical and basic cancer research, with more than $7.3 million in National Cancer Institute research support and 47,000 square feet of space devoted to cancer research. It is a recognized center of excellence in angiogenesis, cellular/molecular immunology, cancer genetics, and molecular signaling research.
Source-Newswise