Can stem cell therapy cure fistula? Yes, treatment with stem cells has had a success rate when used in perianal fistulas due to Crohn’s Disease.
‘Stem cell-based therapy would be easy to implant in the operating room and offer a new option for perianal fistula in Crohn’s disease.’





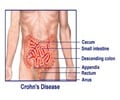
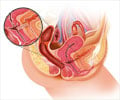
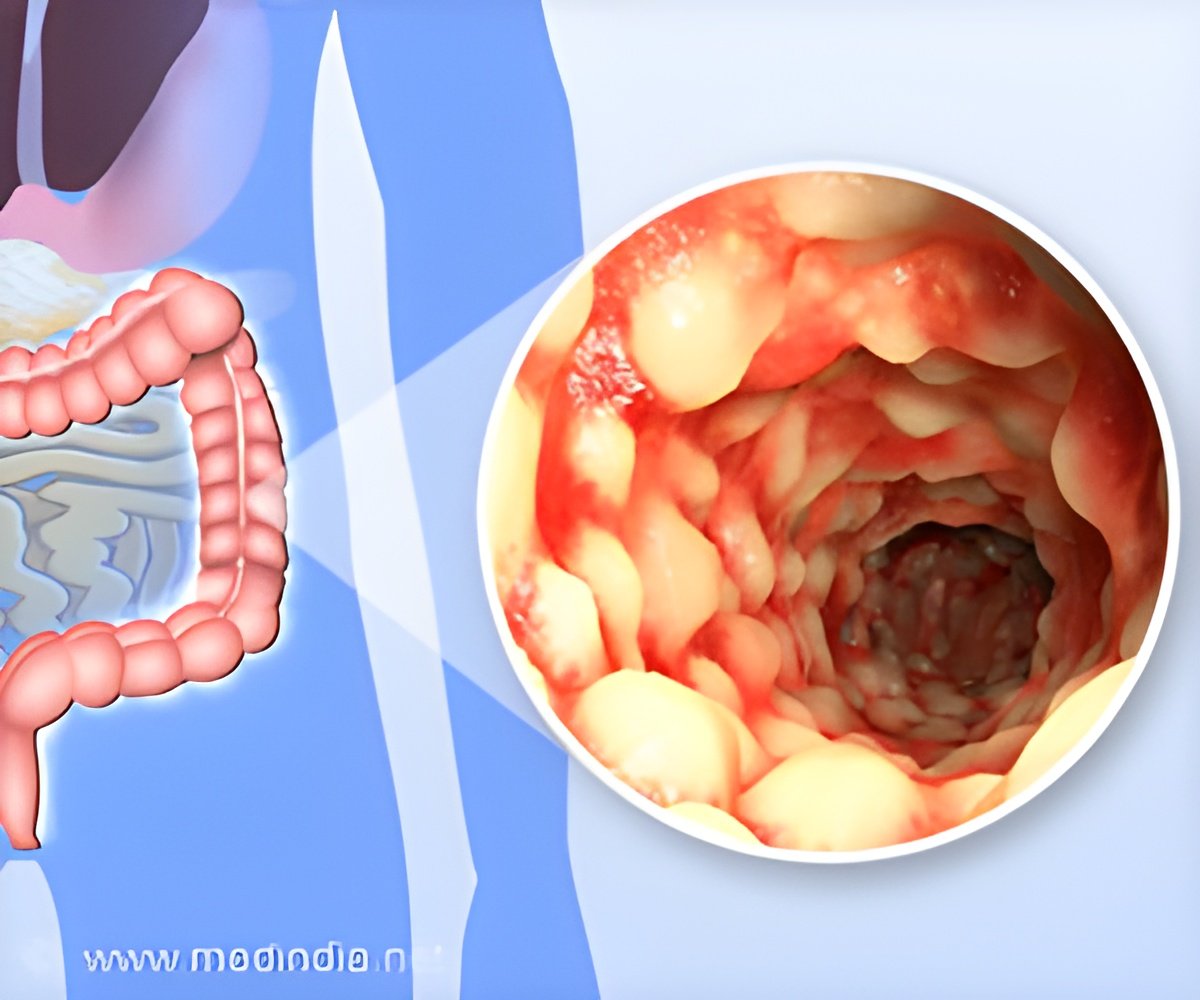
Researchers loaded stem cells from a patient's fat tissue onto a bioabsorbable plug that was then surgically implanted to close the anal fistula tract. They followed the patients for one year and reported the results of their early research.As many as 26% of people with Crohn's disease will develop perianal fistulas. Most often, it starts with an infection within the anal gland and often progresses into an abscess that sometimes requires surgery.
Left untreated, perianal fistulas leak fecal material and can lead to permanent colostomy and, in some cases, cancer. A colostomy is a surgical opening in the abdomen that bypasses the damaged colon to rid the body of solid waste. Perianal fistulas can cause quality-of-life challenges, such as the need to wear pads to protect clothing and prevent odor.
Perianal fistulas are a complex medical condition that even when repaired surgically, can reoccur, causing a lot of suffering for patients. The research team extracted mesenchymal stem cells from the adipose (fat) tissue of 20 patients with perianal fistulas who had not responded to standard medical or surgical treatment.
Stem Cell Therapy: A Crohn's Disease Breakthrough in 2023?
Mesenchymal stem cells are adult stem cells with healing potential that have been well studied. After multiplying the stem cells in the lab, the team combined the cells with a plug created from a dissolvable material.They surgically implanted the plug to close the anal fistula tract and then monitored the patients seven times within 12 months, with a focus on investigating safety. They also studied whether the treatment intervention led to clinical healing that could be confirmed through deep-tissue imaging.
Advertisement
Based on their findings, it is recommended to continue further study of the stem cell-coated fistula plug with larger sample sizes and more types of fistulas. If all goes well, it could take two or three years before this procedure is approved for routine clinical care.
Source-Eurekalert