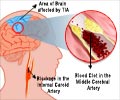
Setting a stent in a vital artery in the neck is a lot safer in patients ineligible for the standard surgical treatment of carotid artery disease, states study in Journal of Vascular Surgery.

"Recent improvements in devices designed specifically for carotid artery stenting have resulted in safer procedures and better clinical outcomes," says Matsumura. "These technological advances, combined with a stronger understanding of patient risk factors, have also improved our ability to select which patients are best suited for the less invasive procedure."
The PROTECT study was a prospective, multi-center clinical trial designed to examine the safety of Emboshield Pro—an embolic protection device used in combination with stenting to help enlarge the blocked artery and capture any plaque that could dislodge during the process. The device is manufactured by Abbott Vascular, which also funded the research. Findings were peer-reviewed before publication.
The study enrolled a total of 322 patients with carotid artery stenosis at 38 centers across the United States between 2006 and 2008. The first 220 patients received a stent and Emboshield Pro, while the other 102 received a stent and an older, now obsolete embolic protection device called Emboshield BareWire. Researchers tracked the composite of clinical outcomes, including death, stroke or myocardial infarction (DSMI) up to 30-days post procedure. Of the 220 who were treated with Emboshield Pro, three patients had a minor stroke (1.4 %), one had a major stroke (0.5%), and one had a myocardial infarction (0.5%), for an overall DSMI rate of 2.3 percent, the lowest rate of complications ever recorded for the procedure in similar multicenter high-risk patient populations.
"These results are consistent with the trends from other trials, where we are seeing complication rates dropping for patients being treated with stents and embolic protection devices," Matsumura says. "This is very good news for high-risk patients suffering with carotid artery disease, who only a decade ago had limited treatment options and poor long-term outcomes."
The US Food and Drug Administration approved carotid artery stenting for high-risk surgical patients in 2004, and recently approved it for standard-risk patients in May 2011. The procedure, which is considered less invasive than carotid endarterectomy, involves a catheter being inserted in the femoral artery of the groin and guided via x-ray to the blocked artery in the neck, where a small balloon is inflated to reopen the vessel. Embolic protection devices work in tandem with stents to help support the newly opened artery and to catch any plaque that could break off during the process and cause a stroke. Strokes affect at least 731,000 people a year in the United States alone, with as many as 25 percent of them being caused by carotid artery disease.
Advertisement