New studies link weight loss drugs to an increased risk of gastroparesis, a serious stomach paralysis condition, highlighting potential side effects of GLP-1 agonists.
Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss
Go to source).
Increased Risk of Gastroparesis in GLP-1 Users
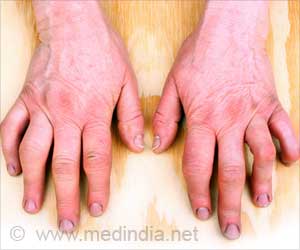
The new data, presented at the Digestive Disease Week 2024 conference, show that the risk of gastroparesis is significantly higher in individuals taking GLP-1 agonists compared to those who are not. Researchers from University Hospitals in Cleveland conducted a study using the TriNetX database, which includes millions of patient records. They focused on obese adults without a prior diagnosis of diabetes or gastroparesis and found that those prescribed GLP-1 medications had a 52% increased risk of being diagnosed with stomach paralysis. Specifically, 10 out of every 10,000 patients on these medications developed gastroparesis, compared to 4 out of 10,000 who were not on the drugs.‘GLP-1 agonists for weight loss and diabetes increase gastroparesis risk by up to 66%. #healthalert #gastroparesisrisk #medindia’





A second study by the University of Kansas corroborated these findings, using records from nearly 300,000 patients. This study found that individuals on GLP-1 medications were 66% more likely to be diagnosed with gastroparesis. Moreover, the incidence of other gastrointestinal issues, such as nausea, vomiting, and gastroesophageal reflux disease (GERD), was also higher among those taking GLP-1 agonists.Ensuring Safety Amidst Rising Usage
Despite the widespread use and efficacy of GLP-1 agonists, such as Wegovy, Ozempic, and Victoza, in promoting weight loss and managing diabetes, these studies raise questions about the thoroughness of clinical trials. Dr. Prateek Sharma from the University of Kansas highlighted that the adverse event of gastroparesis might have been missed due to the relatively small sample sizes of clinical trials. Larger databases and real-world data are necessary to uncover rare side effects like gastroparesis.Furthermore, Dr. Michael Camilleri from the Mayo Clinic noted that the methods used in pharmaceutical trials to assess gastric emptying might have been inadequate. These trials typically use the acetaminophen absorption test, which measures how quickly liquids leave the stomach but does not accurately reflect the emptying of solids—a crucial factor in diagnosing gastroparesis.
The studies emphasize that while GLP-1 agonists are effective, patients and healthcare providers should be aware of the potential gastrointestinal side effects, including the risk of developing gastroparesis. Monitoring and managing these side effects are essential, especially given that some patients have reported prolonged symptoms even after discontinuing the medication.
As the use of GLP-1 agonists continues to rise, with 25,000 new users starting Wegovy each week in the U.S. alone, it is vital to balance their benefits against the risks and to ensure that patients are fully informed about potential side effects. Further research and vigilance in clinical practice are necessary to optimize the safety and efficacy of these medications.
Advertisement
- Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss - (https://jamanetwork.com/journals/jama/fullarticle/2810542)
Source-Medindia