LIPUS was approved for fracture healing by the US Food and Drug Administration (FDA) in 1994.

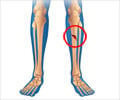
‘Every year around 4 in 100 people of all ages have a fracture - and up to 10% of these experience slow or complicated healing. As such, fractures have been a target for numerous interventions to aid recovery.’





Their advice is part of The BMJ's 'Rapid Recommendations' initiative - to produce rapid and trustworthy guidance based on new evidence to help doctors make better decisions with their patients. Both the new evidence and the guidance are published by The BMJ. Every year around 4 in 100 people of all ages have a fracture - and up to 10% of these experience slow or complicated healing. As such, fractures have been a target for numerous interventions to aid recovery.
LIPUS was approved for fracture healing by the US Food and Drug Administration (FDA) in 1994 and is also supported by the UK National Institute for Health and Care Excellence (NICE).
Each device costs between US$1300 and $5000 and data suggest it is commonly used in clinical practice. But some studies have shown that the potential benefits of LIPUS on bone healing are highly uncertain.
So The BMJ's guideline panel - made up of bone surgeons, physiotherapists, clinicians and patients with experience of fractures - carried out a detailed analysis of the latest evidence.
Advertisement
As such, they unanimously recommend against LIPUS for patients with any bone fractures or osteotomy (the surgical cutting of a bone to allow realignment).
Advertisement
It is unlikely that new trials will alter the evidence, they add. And they suggest that future research "should focus on other interventions that have a greater probability to speed up healing."
Source-Eurekalert