A new study has revealed that a new cervical cancer drug offers the first good hope of extending life for women with advanced stages of the disease.
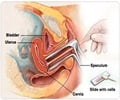
That's why early screening is so critical -- regular Pap smears have managed to reduce deaths in wealthy countries by 80 percent.
"Women with advanced cervical cancer don't have many options," said lead study author Krishnansu Sujata Tewari, a professor of obstetrics and gynecology at the University of California Irvine.
"We finally have a drug that helps women live longer."
The study found that women who were given the drug bevacizumab (Avastin) along with their chemotherapy prolonged survival to an average of 17 months, compared with 13.3 months for those who only received chemotherapy.
The phase III clinical trial of 452 women found that 48 percent of patients who received the drug saw their tumors shrink, compared with 36 percent of those who did not.
Advertisement
The study was presented at the American Society of Clinical Oncology's annual meeting in Chicago.
Advertisement
Genentech's drug bevacizumab is currently approved by US regulators for use in several advanced cancers, but has not yet been approved for gynecological cancer. It works to block blood vessel formation in the tumor.
Tewari said he hopes the results are strong enough to gain approval for the drug to be used with cervical cancer patients.
"I'm hoping this is a definitive study and it will change practices," he told reporters at a press conference announcing the results.
"This is also possibly a first step toward turning cervical cancer into a chronic disease, helping women live longer and allowing time for additional treatments that could further slow the cancer's progression and improve survival."
Thanks to early detection and treatment, cervical cancer only kills 4,000 women a year in the United States.
But for those who do not respond to treatment, the situation is grim.
"Women die in the prime years of their lives and children lose their mothers," Tewari said.
Bevacizumab is likely too costly to help the bulk of advanced cervical cancer patients who live in developing countries, Tewari acknowledged.
Help could nonetheless eventually reach them now that scientists know that blocking blood vessel formation can impact advanced cervical cancer.
"It really opens up doors to study other classes of drugs," he told reporters.
A second study released Sunday offered hope to women in developing countries who rarely have access to early screening.
Researchers in India were able to reduce cervical cancer rates by 31 percent by using a simple, cheap vinegar test that can be administered by community health workers and delivers instant results.
Source-AFP