A mutant gene long thought to accelerate tumor growth in thyroid cancer patients actually inhibits the spread of malignant cells, a Mayo Clinic study, led by an Indian-origin researcher, has found.
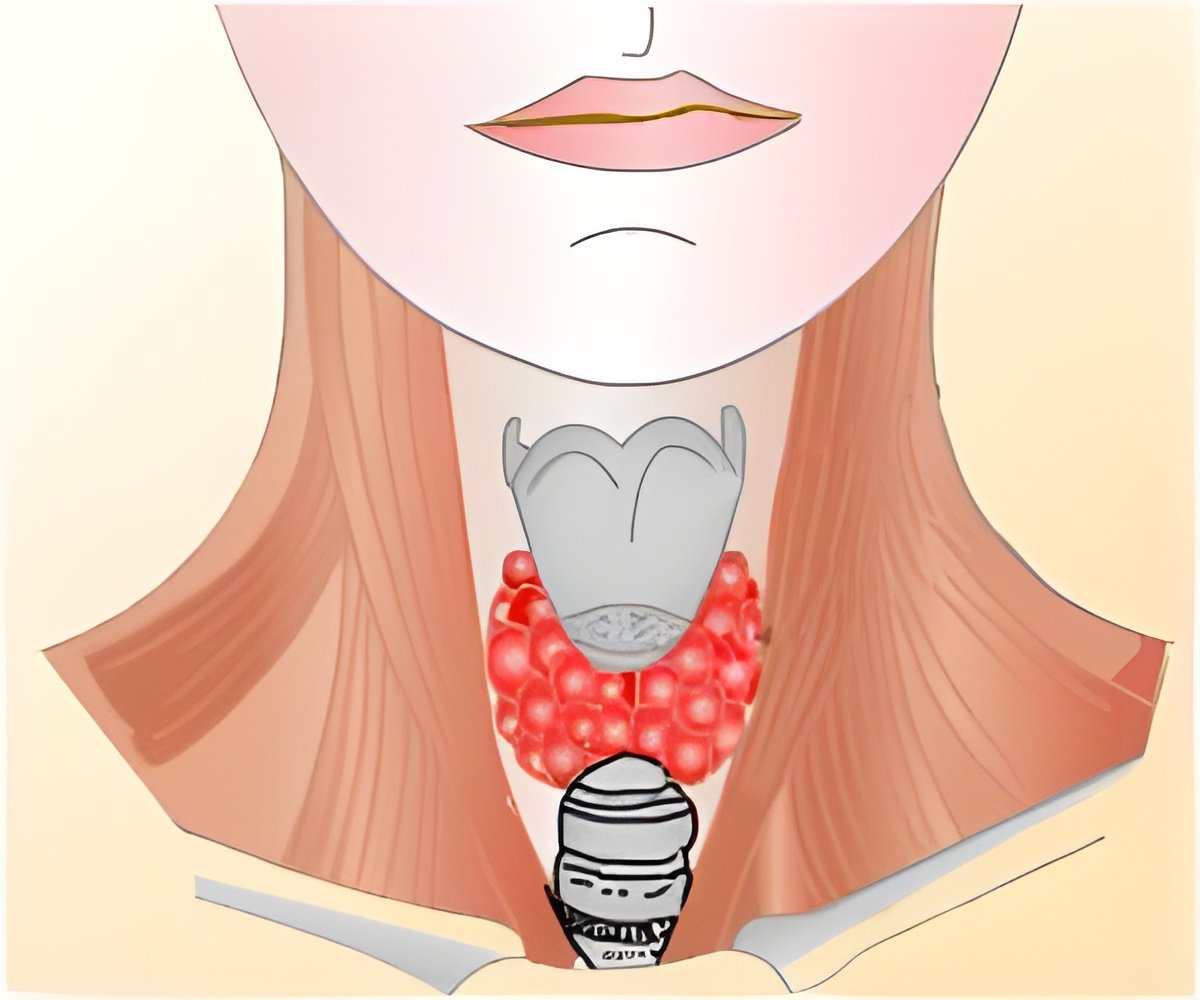
Reddi's research found that the PAX8/PPAR? fusion protein, developed from a mutated fusion gene found in many follicular thyroid carcinomas, functions as a tumor suppressor by upregulating (encourages natural production of) microRNA-122 and PTEN, both naturally occurring anti-tumor agents.
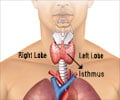
PAX8/PPAR? results from the translocation of genetic material between human chromosomes 2 and 3.
Previous in vitro studies of the PAX8/PPAR? protein found rapid acceleration of cell growth, which led researchers to the false interpretation that PAX8/PPAR? functioned as an oncogene, a type of mutated gene that encourages tumor propagation, said Reddi .
But Mayo Clinic's in vivo animal studies showed that PAX8/PPAR? upregulates the well-known anti-cancer protein PTEN, as well as microRNA-122, and likely facilitates other cancer-fighting molecules.
PAX8/PPAR? does not boost tumor progression when exposed to cancerous cells. Rather, its facilitation of other native anti-cancer molecules appears to outweigh the tumor propagation, said Reddi.
Advertisement
The findings will be presented at the Endocrine Society meeting in Boston.
Advertisement