Researchers are currently tracking previously unknown movements of a set of specialized cells to analyze how the immune system is able to mount a successful defense against hostile invaders.
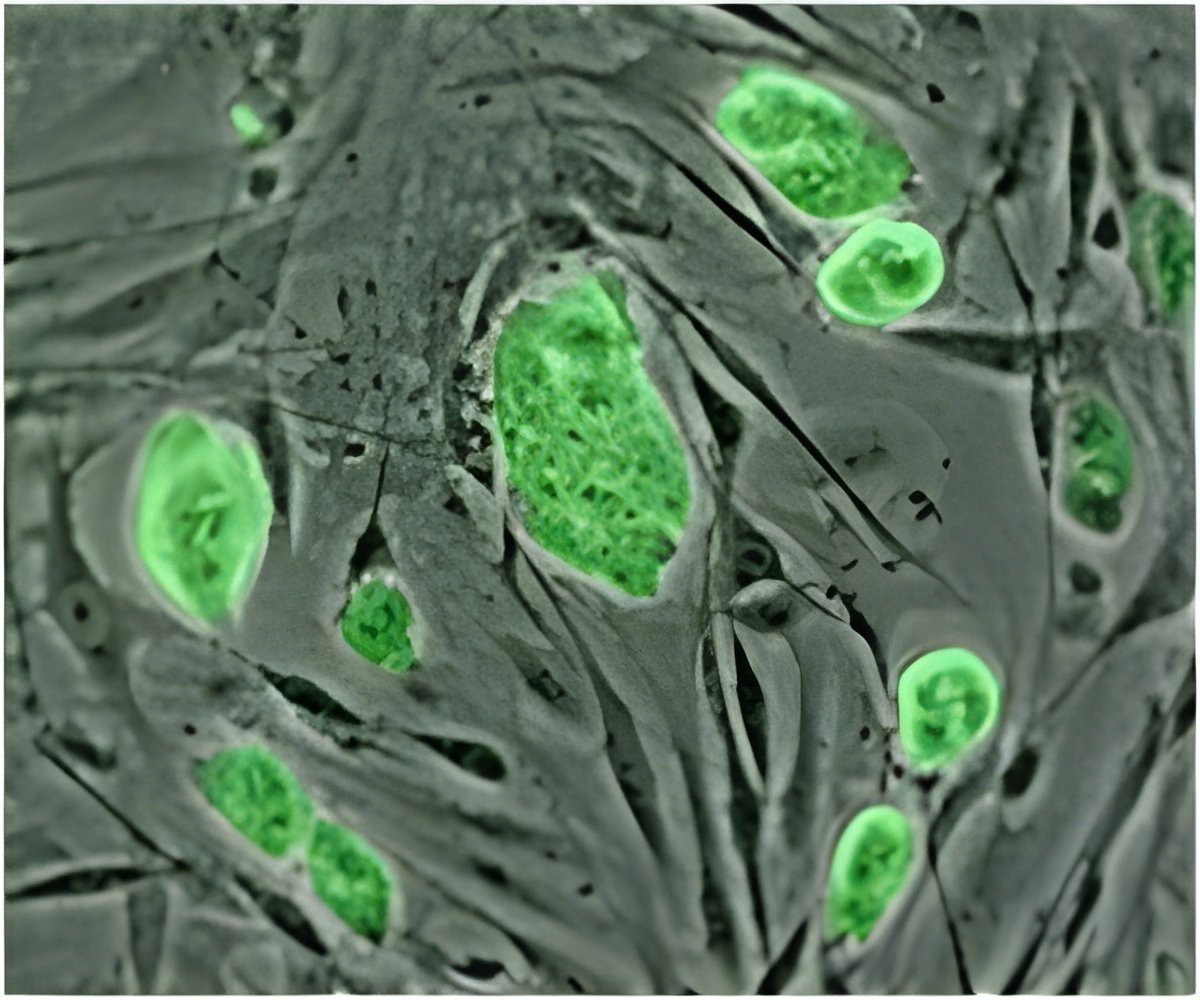
During the mutation cycles, most of the B cells in any given GC fail to achieve sufficient antibody affinity and are eliminated. A few, however, measure up, and are selected to proliferate, leave the GC, and produce the antibodies that attack the offending pathogen. This selection process is driven by cells known as T follicular helper (Tfh) cells, which essentially cherry-pick the B cells capable of producing the most potent antibodies. Yet much of the behavior of Tfh cells has been something of a mystery. Until now.
By developing and deploying a novel cellular tagging and imaging methodology, a team of scientists led by Whitehead Fellow Gabriel Victora has discovered that, unlike their B cell counterparts, Tfh cells continually move from GC to GC within a lymph node. This surprising finding is reported in the August 9th edition of the journal Science.
Victora used fluorescence microscopy to trace the Tfh cells' migratory patterns in the lymph nodes of living mice, noting that these cells constantly migrate between germinal centers. This activity may enhance the diversity of antibody production by introducing static B cells to a range of dynamic Tfh cells, and could help explain how the immune system copes with mutating pathogens.
"We think this could be the way our immune system can maintain a targeted immune response, even when the target is moving," says Victora, whose latest research was conducted with collaborators at the Rockefeller University in New York.
Victora believes that continued mapping of the choreography between B cells and Tfh cells has significant implications for improving vaccine development, as it suggests opportunities to influence antibody development.
Advertisement
Advertisement