Beta-amyloid – a pathological hallmark protein of Alzheimer's disease adopts a highly disordered shape in a matter of microseconds.
‘Beta-amyloid – a pathological hallmark protein of Alzheimer's disease adopts a highly disordered shape in a matter of microseconds. This adds on to the need for a powerful tool to investigate these classes of proteins with fast and disordered motions for an effective treatment.’





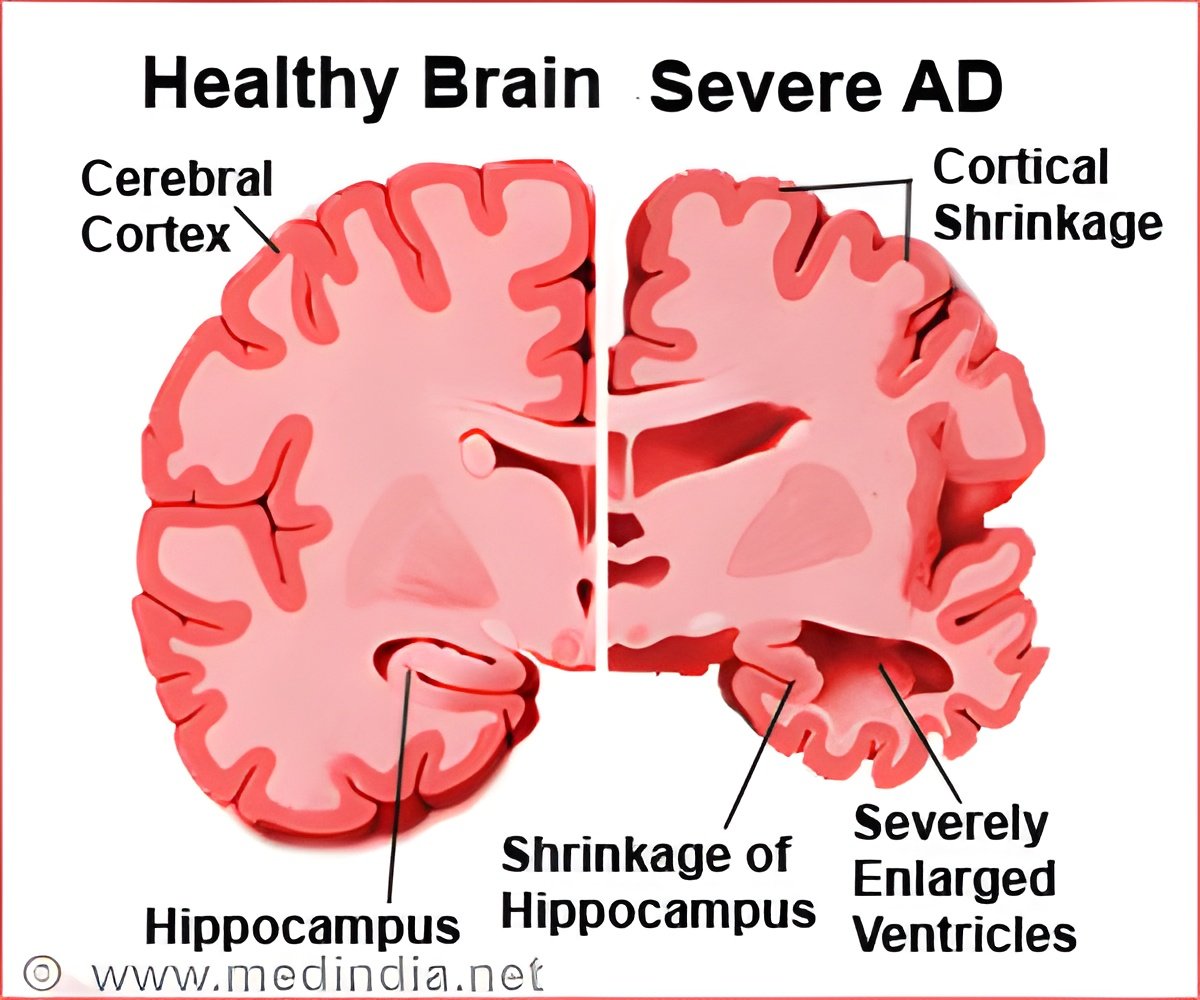
Alzheimer's disease (AD) is a neurodegenerative disease that leads to gradual memory loss and behavioral changes. It is characterized by the formation of beta-amyloid plaques and the tau proteins in the brain tissues, long before the actual symptoms occur. "We are used to thinking of proteins as molecules that fold into well-defined structures: finding out how this process happens has been a major research focus over the last 50 years. However, about a third of the proteins in our body do not fold, and instead remain in disordered shapes, sort of like noodles in a soup", says Professor Michele Vendruscolo from Cambridge's Centre for Misfolding Diseases, who led the research.
The Dynamic pathology of Alzheimer’s Disease
Traditional research methods address the problem of determining static structures, not structures in motion. The approach developed by the present study harnesses the power of Google's computer network to generate large numbers of short trajectories, that is, to define the transition frequencies by which disordered proteins jump between different shapes.
The team studied a variant of amyloid beta in which one of the amino acids is modified by oxidation. It was found that amyloid beta hops between widely different states millions of times per second without ever stopping in any particular state.
Advertisement
"By making disordered proteins even more disordered, we can prevent them from self-associating in aberrant manners," says Vendruscolo.
Advertisement
Source-Medindia











