Applied Data, a healthcare therapeutics firm has concluded its study on the development of technology in the field of implantable glucose monitors.
Applied Data, a healthcare therapeutics firm has concluded its study on the development of technology in the field of implantable glucose monitors.
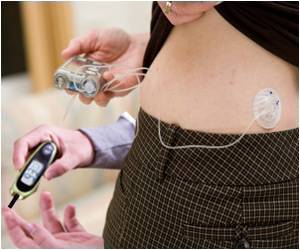
According to them, there has been a massive spurt in new ideas and innovations in this field ever since 1991, when the FDA approved the first implantable glucose monitor for Type 1 diabetics.The last decade has seen the device move from the abdomen to the arm, then forehand and even the wrist.
There have been other notable changes such as the recent real–time measuring of glucose levels. These are conveyed to a monitor via sensing from the implanted device.
Yet, says Applied Data, technology is yet to replace the old-fashioned finger prick method.
Problems with the current devices include 'sensor drift' as well as limited life span of the device. Technologists are working to increase its life span from few days to a month or more, or even up to a year.
Says Applied Data, 'The solutions to these challenges are being pursued on a number of fronts, and will require advances in biomaterials, embedded electronics, packaging, and power management. But these improvements will be evolutionary, rather than revolutionary. The bottom line is that, after years of disappointment, this time the new age in diabetes management should arrive on schedule.'
Advertisement
Yet hopes are high for diabetics round the world that technology will manufacture a product that will out-date the present 'blood–drawing' method of glucose monitoring.
Advertisement
ANN