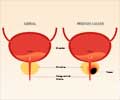
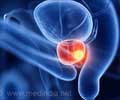
The US federal regulators are now investigating products containing testosterone after some recent studies link a higher risk of heart attack and stroke in men who are treated with the hormone

"FDA is providing this alert while it continues to evaluate the information from these studies and other available data," it said in a safety alert, referring to two related studies.
Patients undergoing testosterone therapy should not stop their treatment without consulting their physician first, the FDA recommended.
Health care professionals were also asked to consider whether the benefits of FDA-approved testosterone treatment were likely to outweigh the possible risk of treatment.
The announcement followed publication of a study on Wednesday by the PLOS ONE science journal suggesting that men aged 65 and older being treated with testosterone were twice as likely to suffer heart attacks in the month after they began treatment.
The study, which analyzed findings from 56,000 men in the United States between 2008 and 2010, also revealed a sharply increased risk among younger men with a history of heart disease.
Advertisement
A US government-funded study to determine whether men using testosterone gel to build muscle and increase strength was halted in 2009 after the high rate of cardiovascular problems related to the treatment.
Advertisement