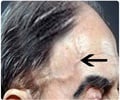
FDA grants break through therapy designation for Roche’s Tocilizumab drug which is used in the treatment of Giant Cell Arthritis.

‘Giant Cell Arteritis drug receives features of fast track designation from FDA for the development of the drug.’





Tocilizumab from Roche has received prior approval in 2010 and the company continues to research and find new uses for the treatment of autoimmune diseases.Break Through Therapy Designation from FDA is given for the treatment of life threatening diseases or symptoms. It is usually adopted as a part of FDA Safety and Innovation Act (FDASIA). This designation is given for the development of new drug which has preliminary clinical evidence demonstrating substantial improvement on atleast one existing therapy. Tocilizumab is the fifth best selling medicine of Roche which is prescribed for the treatment for rheumatoid and juvenile idiopathic arthritis. Sandra Horning, Chief Medical Officer said "The FDA Breakthrough Therapy designation for giant cell arteritis underscores our continued commitment to explore Actemra/RoActemra in autoimmune diseases with significant unmet need"
She also added that this drug would be the first new treatment for the condition in 50 years.
The company also said that Tocilizumab combined with six month steroid therapy were found to be more effective through the year compared to the standard 6 or 12 month steroid therapy.
Advertisement