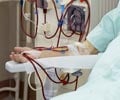
Tolvaptan slows down the formation of kidney cysts and reduces the decline in kidney function. This postpones the time-limit for dialysis or a transplant.
- Tolvaptan in its clinical trials that lasted over a period of one year, in patients with later-stage autosomal dominant polycystic kidney disease showed a decline in kidney damage.
- Autosomal dominant polycystic kidney damage (ADPKD) is one of the most common and potentially deadly forms of polycystic kidney disease; it is known to affect many people in a single family.
- The requirement of dialysis or a kidney transplant can either be delayed or postponed by taking tolvaptan therapy in patients suffering from ADPKD.
Tolvaptan Facts
Tolvaptan belongs to a new class of medication called ‘vaptan’ drugs which blocks the action of vasopressin receptors. Tolvaptan is the first available oral vasopressin receptor 2 antagonist which was approved by US FDA in 2009 to be most commonly used in treating hypervolemia (excess fluid retention) and hyponatremia (low blood sodium levels) in patients associated with heart failure, cirrhosis, and syndrome of inappropriate antidiuretic hormone (SIADH). However, FDA recommends that tolvaptan should not be used for more than 1 month and it is not a drug of choice in patients with liver ailments as tolvaptan may result in serious liver injury leading to liver failure.The Scottish Medicines Consortium (SMC) approved the use of tolvaptan in adults with ADPKD followed by National Institute for Care and Clinical Excellence’s (NICE) in 2015 that approved its use in England and Wales. Prior to the approval of tolvaptan, there was no licensed drug to treat ADPKD in the United Kingdom.
Disease-Modifying Treatment for ADPKD with Tolvaptan
Tolvaptan underwent a phase 3, randomized, multicenter, placebo-controlled, double-blind trial compared with a placebo.Tolvaptan has been administered at an average dose of 95mg/day for a three-year duration. The change in the estimated glomerular filtration rate (GFR) and the aminotransferase level was recorded in the tolvaptan and the placebo group.
At the end of the trial, it was found that tolvaptan slowed the usual increase in kidney volume by 50% and decreased the decline in kidney function by 30% in the estimated GFR when compared to placebo during a 12-month duration in patients with later-stage autosomal dominant polycystic kidney disease.
The results of the study deliver the necessity for a dialysis or a kidney transplant can be postponed in patients with later-stage ADPKD, thereby increasing the quality of life.
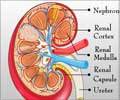
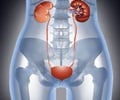
Autosomal Dominant Polycystic Kidney Disease (ADPKD)
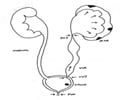
ADPKD is a genetic disorder which is a painful form of inherited polycystic kidney disease. In this disease, fluid-filled cysts continue to develop and enlarge in the kidneys eventually leading to kidney failure.In some cases, the affected polycystic kidneys are as large as the size of a football and each kidney weighs around 30 pounds. The cysts may spread to other organs such as liver or pancreas or seminal vesicles. Nearly 50% of patients with ADPKD develop end-stage renal disease and require either dialysis or kidney transplant.
Diagnosis of ADPKD can be done by performing ultrasound, CT or MRI scan. DNA testing such as gene linkage testing or direct DNA sequencing can also be carried out to study the family health status but come with the disadvantages of being costly procedures.
Symptoms of AKPKD
- Pain in the kidneys
- High blood pressure
- Urinary tract infection
- Blood while passing urine
- Kidney stones
References:
- Vicente E. Torres, Arlene B. Chapman, Olivier Devuyst, Ron T. Gansevoort, Ronald D. Perrone, Gary Koch, John Ouyang, Robert D. McQuade, Jaime D. Blais, Frank S. Czerwiec, Olga Sergeyeva. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. New England Journal of Medicine, 2017; DOI: 10.1056/NEJMoa1710030
- Tolvaptan Phase 3 Efficacy and Safety Study in Autosomal Dominant Polycystic Kidney Disease (ADPKD) (TEMPO3:4) - (https://clinicaltrials.gov/ct2/show/NCT00428948)
- Tolvaptan - (https://en.wikipedia.org/wiki/Tolvaptan)
- What is ADPKD? - (https://pkdcure.org/what-is-pkd/adpkd/)
- Tolvaptan now recommended UK-wide for the treatment of ADPKD - (https://www.kidneyresearchuk.org/news/tolvaptan)
- Tolvaptan - (https://medlineplus.gov/druginfo/meds/a609033.html)
- What is ADPKD? - (http://www.pkdinternational.org/what-is-pkd/adpkd/)