Torax Medical (Shoreview, Minnesota) has received Food and Drug Administration (FDA) approval for its FENIX continence restoration system.
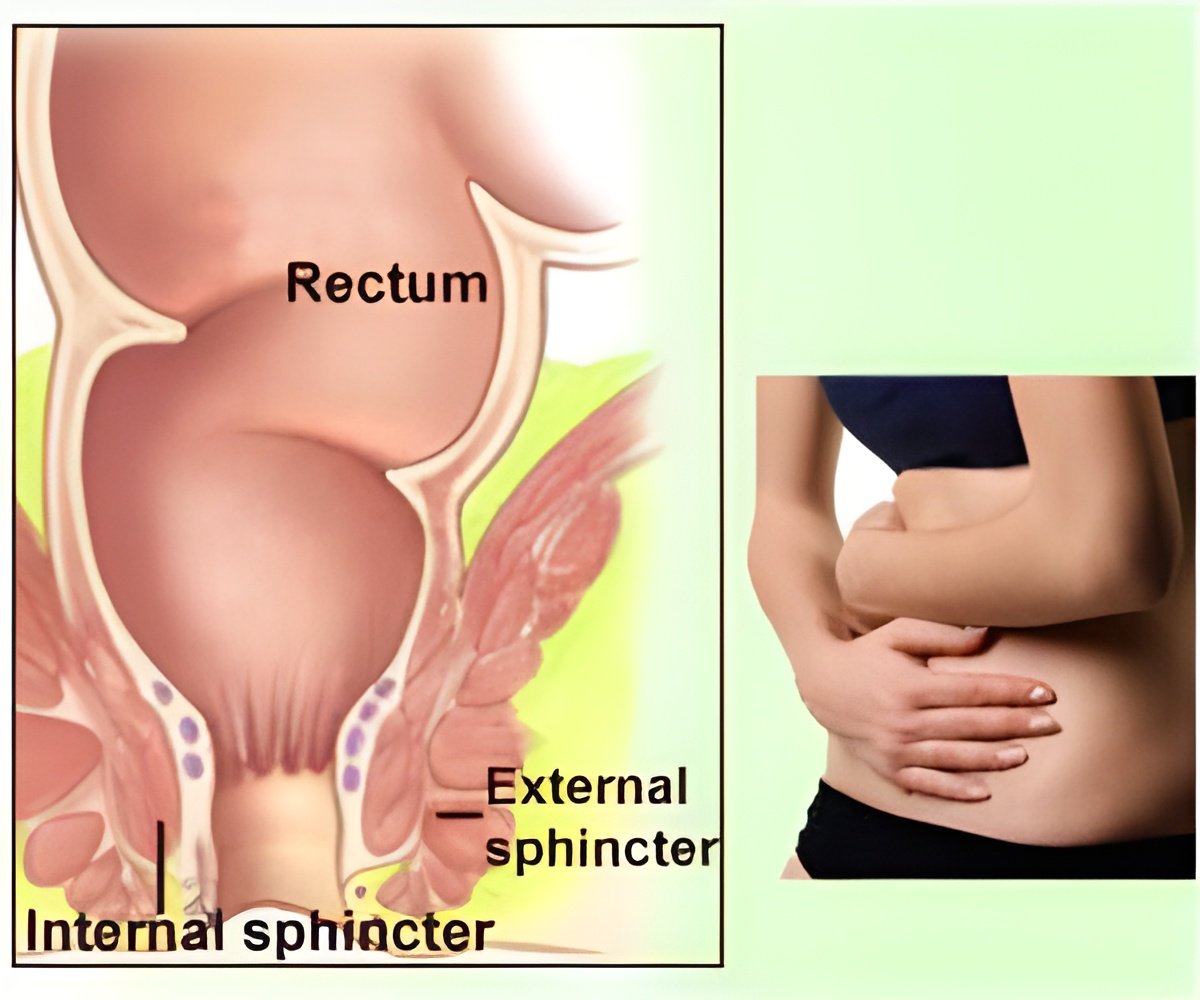
‘Torax Medical’s FENIX continence restoration system prevents involuntary discharge toward the other end of the gastrointestinal tract. The device is implanted using a single incision.’





However, the FENIX prevents involuntary discharge toward the other end of the gastrointestinal tract. The device is implanted using a single incision. The size of the bracelet is decided using a provided tool. X-rays are used to confirm the appropriate size of the implant and to make sure the functionality of the system following the procedure. Fecal incontinence or bowel incontinence is a lack of control over defecation, leading to involuntary loss of bowel contents - including flatus (gas), liquid stool elements and mucus, or solid feces. Fecal incontinence is a sign or a symptom, not a diagnosis.
Source-Medindia