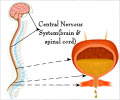
Researchers will use a technique known as (TESCoN), a noninvasive therapy that delivers low-intensity electric impulses to the spinal cord. The trial used spinal cord stimulation that earlier research suggests might treat bladder dysfunction.
Researchers will use a technique known as Transcutaneous Electrical Spinal Cord Neuromodulation (TESCoN), a noninvasive therapy that delivers low-intensity electric impulses to the spinal cord.
The trial follows a recent study published in Frontiers in Systems Neuroscience led by Evgeniy Kreydin, MD, a Keck Medicine urologist and assistant professor of clinical urology at the Keck School of Medicine of USC. Kreydin and colleagues treated 14 patients with bladder dysfunction due to either spinal cord injury, stroke, multiple sclerosis or idiopathic cause with spinal cord stimulation sessions three times a week for eight weeks. All patients reported improved bladder sensation as well as a reduced number of incontinence episodes and night-time bladder voiding.
An overactive bladder causes several urological problems, such as frequent urination and incontinence. Existing treatments for the condition can cause adverse side-effects or require invasive, highly specialized procedures.
"TESCoN is well-tolerated by patients and easy for doctors to administer," says Kreydin, who will be co-investigating the trial along with Keck Medicine urologist David Ginsberg, MD, a professor of clinical urology at the Keck School.
In this double-blinded, sham-controlled trial, half the participants will receive two one-hour sessions of TESCoN per week over 12 weeks. The stimulation will be applied via electrodes attached to a device that emits low-intensity impulses through adhesive pads placed on the patient’s back. The other half of the participants will receive a placebo, or sham stimulation over course of the trial.
Advertisement
TESCoN is developed by the medical device company spineX. Company founders will be initiating discussions with the U.S. Food and Drug Administration to gain regulatory approval for the procedure.
Advertisement
"The ultimate goal of the trial is to improve peoples’ sense of well-being," says Kreydin. "An overactive bladder can cause discomfort, inconvenience and embarrassment. The more control patients have over their bladders, the more control they have over their lives."
Source-Eurekalert