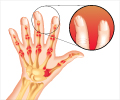
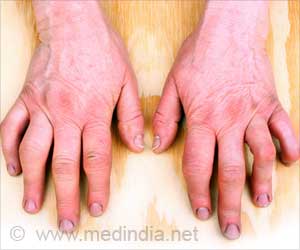
Janssen, a subsidiary of Johnson and Johnson (J&J), recently announced that Tremfya (guselkumab) received Food and Drug Administration (FDA) approval for patients with active psoriatic arthritis (PsA).
‘The US F.D.A approved Tremfya for patients with active psoriatic arthritis. It is having a comparable safety profile to IL-17 inhibitors.’
Read More..




Tremfya beat out competing IL-23s: AbbVie’s Skyrizi (risankizumab) and SunPharma’s Ilumya/Ilumetri. The selective IL-23 inhibitors represent the most promising pipeline drug class for PsA. Read More..
Tiffany Chan, Immunology Analyst at GlobalData, explains: “Tremfya is expected to do well, based on J&J’s previous experience in the disease space marketing Stelara. Since Stelara’s patent is set to expire in 2023 in the US and Japan and in 2024 in the *5EU, it is likely that J&J will be able to preserve its position in the PsA space with Tremfya’s launch.”
Tremfya and the other selective IL-23 inhibitors is likely to also compete with the IL-17 inhibitors, Eli Lilly’s Taltz (ixekizumab) and Novartis’ Cosentyx (secukinumab), as second-line biologics for PsA patients. In head-to-head trials in plaque psoriasis, Tremfya was proven to be superior to Cosentyx but inferior to Taltz in skin clearance, from the IXORA-R and ECLIPSE trials, respectively.
The medication appears to have a comparable safety profile to the IL-17 inhibitors but a better safety profile compared to Stelara, an IL-12 and IL-23 dual inhibitor.
Notably, unlike their IL-17 counterparts, the IL-23 inhibitors are not contraindicated in patients with inflammatory bowel disease, which is a significant co-morbidity for PsA patients. Taken as a whole, Tremfya will pose a formidable challenge to the current marketed therapies.
Advertisement
In the head-to head VOYAGE-2 trial in plaque psoriasis, Tremfya was superior to Humira, measured by the PASI90 criteria.
Source-Medindia