A 3D hydrogel scaffold has been created to transform stem cells into muscle fiber, which can be easy and efficient in tissue repair.
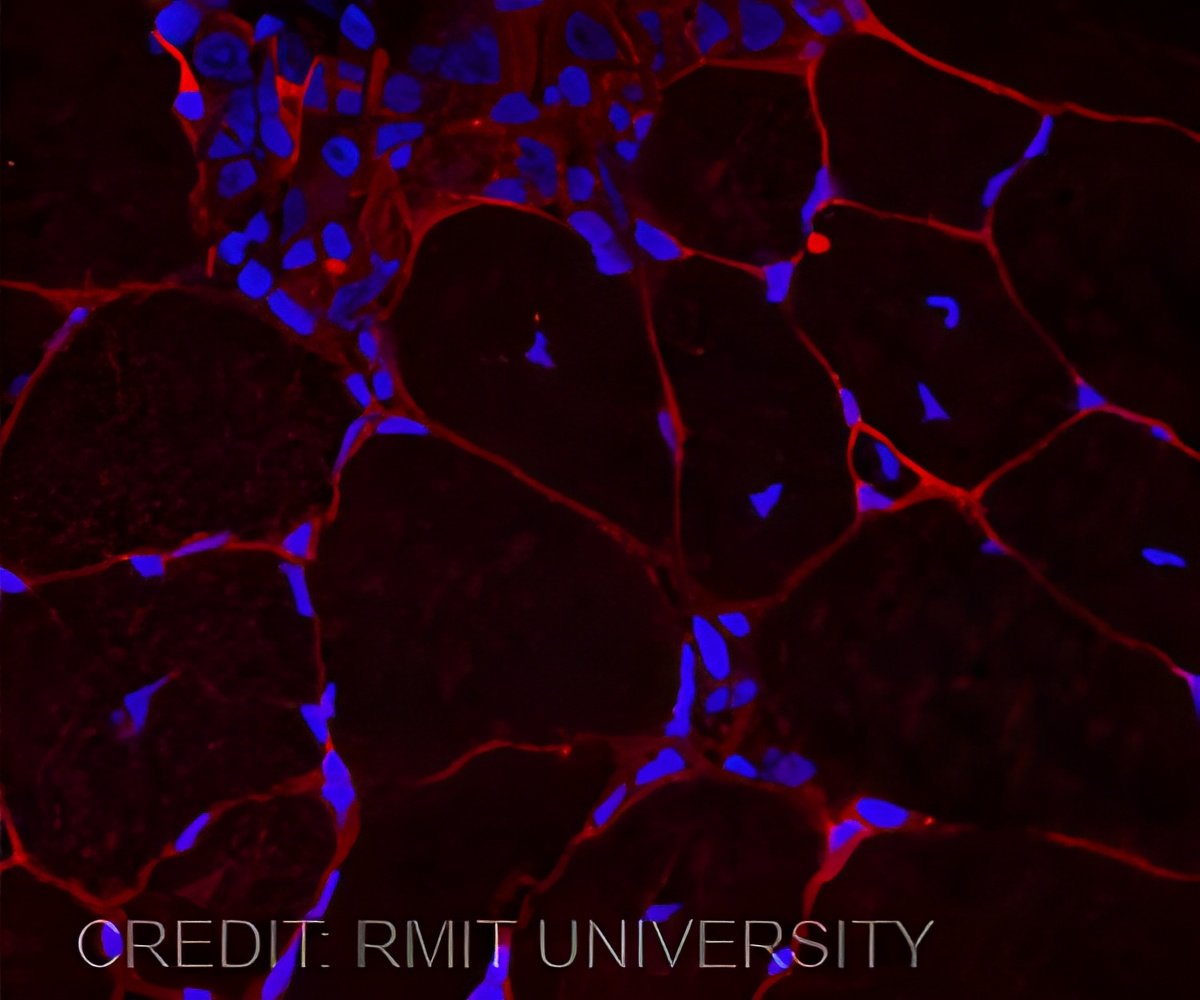
‘Combining a hydrogel with proteins present during embryonic development in a 3D scaffold can help grow muscle.’





The Australian biomedical engineers have for the first time incorporated the natural processes of embryonic development to build a material that can more naturally communicate with stem cells for effective tissue repair.Led by Dr Richard Williams from RMIT University, in Melbourne Australia, the researchers have combined lab-made peptides with natural proteins and polymers to create a 3D "hydrogel scaffold".
The scaffold uses natural mechanisms to incorporate signals found in the natural developmental environment to support and engineer stem cells into muscle fiber.
"Our stem cells are at their best during development, where a scaffold drives them to produce all our tissues and organs; yet as people grow and age, they lose this ability and get a build-up of undesirable structures," Williams said.
"So there is a real need for ways to repair and replace parts of the body as they wear out or become damaged, particularly as our population is now living longer.
Advertisement
"It's like travelling back in time when our potential for tissue development and repair was at its best."
Advertisement
"We have developed a simple, cheap, yet powerful toolkit that encourages different environments to let different tissues grow," he said.
"The material properties of natural tissues, like muscles, are really complex and extremely difficult to mimic synthetically.
"Researchers have been producing 'organizing' signals for years to understand how cells work but only in a flat, or 2D, environment.
"We have found a way to use the proteins present during embryonic development in a 3D scaffold, and use this extra dimension to potentially grow muscle.
"Our research shows that combining a hydrogel with these small proteins is easy and effective. In fact, just 24 to 72 hours after seeding the cells into the tissue, there was a noticeable reorganization of individual cells into the multi-cellular structures that go on to make up functioning muscle."
The RMIT researchers are now exploring how they can bioprint muscle with the stem cells already embedded to put nerves, blood vessels and muscles together into lab grown "spare parts".
Source-Eurekalert