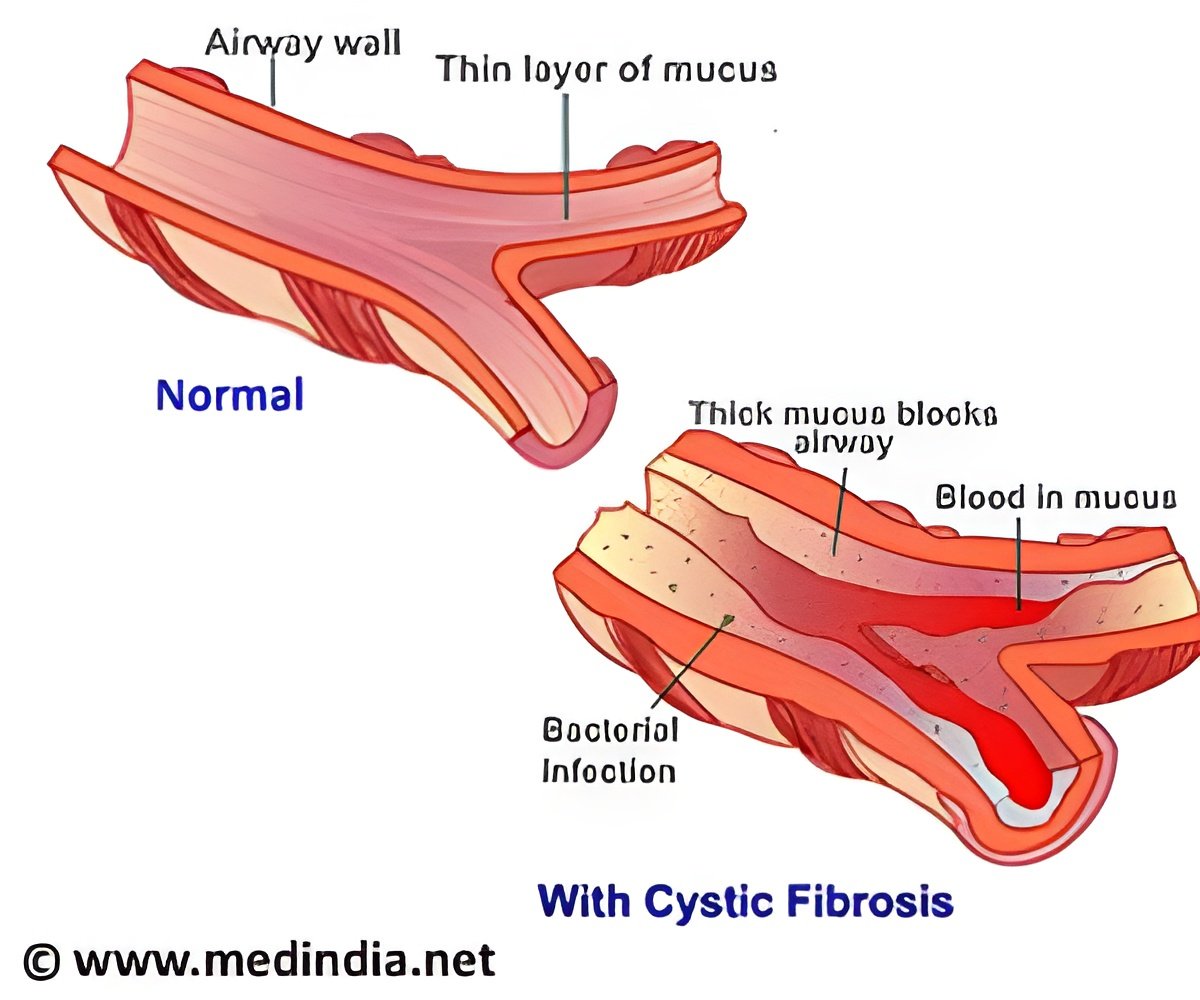
Cystic Fibrosis is a progressive genetic disease that causes persistent lung infections, clogs the airways and limits the ability to breathe over time.
‘Categorizing gene mutations is vital for drug development and treatment.
Orkambi, a drug targets a specific gene mutation and treats about 70% of cystic fibrosis patients.
’





Because of the benefits of early diagnosis and the benefits of early nutritional treatments, mandatory newborn screening for CF is conducted throughout the U.S. and other countries. But with so many mutations identified to date in the CF Transmembrane Conductance Regulator (CFTR) gene - and contributions to the disease undefined for most - providers, including doctors, genetic counselors and worried parents alike, are not always sure to how to interpret screening results. California began newborn screening for CF in July 2007. To address the challenges of screening in such an ethnically diverse state, a three-step model was adopted that includes CFTR DNA sequencing in order to explore the full range of the CF spectrum and help scientists to understand the various mutations and their clinical significance. The three genotype groups created for this study were: those with two CF-causing mutations; those with one mutation of varying clinic consequences (VCC) and those with one mutation of unknown liability (Unknown).
"Our study focused on children who have one CF-causing mutation and a second of varying or unknown clinical consequences, and followed them over time to see which mutations eventually resulted in disease symptoms and those that resulted in no disease," said first author Danieli B. Salinas, MD, of CHLA's Division of Pulmonology.
While most infants with VCC and unknown CFTR mutations do not meet diagnostic criteria for CF, a small proportion do.
The results of the cohort study showed that about 5 percent of VCC and 11 percent of Unknown developed CF. Children carrying Unknown mutations were determined to be at risk, while other mutations were benign.
Advertisement
On the other hand, if parents know that the mutation has been found to be benign, meaning the infant has been screened with a positive genetic profile for CF, but not the disease, it saves them considerable worry, and they know their baby no longer has to be seen for follow-up tests, Salinas added.
Advertisement
Source-Newswise