Disrupting the molecular function of a tumor suppressor causes improper formation of a protective insulating sheath on peripheral nerves, finds new research.
"This study is just the tip of the iceberg and a fundamental discovery because of the unexpected finding that a well-known tumor suppressor gene has a novel and important role in myelinating glial cells," said Biplab Dasgupta PhD, principal investigator and a researcher at the Cincinnati Children's Cancer and Blood Diseases Institute (CBDI). "Additional study is needed, as the function of Lkb1 may have broader implications – not only in normal development, but also in metabolic reprogramming in human pathologies. This includes functional regeneration of axons after injury and demyelinating neuropathies."
The process of myelin sheath formation (called myelination) requires extraordinarily high levels of lipid (fat) synthesis because most of myelin is composed of lipids, according to Dasgupta. Lipids are made from citric acid which is produced in the powerhouse of cells called mitochondria. Success of this sheathing process depends on the cells shifting from a glycolytic to mitochondrial oxidative metabolism that generates citric acid, the authors report.
Dasgupta's research team used Lkb1 mutant mice in the current study. Because the mice did not express Lkb1 in myelin forming glial cells, this allowed scientists to analyze its role in glial cell metabolism and formation of the myelin sheath coating.
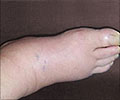
When the function of Lkb1 was disrupted in laboratory mice, it blocked the metabolic shift from glycolytic to mitochondrial metabolism, resulting in a thinner myelin sheath (hypomyelination) of the nerves. This caused muscle atrophy, hind limb dysfunction, peripheral neuropathy and even premature death of these mice, according to the authors.
Peripheral neuropathy involves damage to the peripheral nervous system – which transmits information from the brain and spinal cord (the central nervous system) to other parts of the body, according to the National Institute of Neurological Disorders and Stroke (NINDS). There are more than 100 types of peripheral neuropathy, and damage to the peripheral nervous system interferes with crucial messages from the brain to the rest of the body.
Advertisement
They tested the effect of boosting citric acid levels in the Lbk1 mutant Schwann cells. This enhanced lipid production and partially reversed myelin sheath formation defects in Lbk1 mutant Schwann cells. Dasgupta said this further underscores the importance of Lbk1 and the production of citrate synthase.
Advertisement
Source-Eurekalert