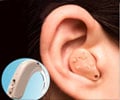
EarLens is different from traditional hearing aids because it uses a laser diode and direct vibration of the eardrum to amplify sound.

The device consists of two parts: a tympanic membrane transducer and a behind-the-ear audio processor. The tympanic membrane transducer is non-surgically placed deeply into the ear canal on the eardrum. And the behind-the-ear audio processor sits on the outer ear and is connected to an ear tip that is placed in the ear canal.
“For the millions of people with hearing impairment, hearing aids can significantly improve regular daily communications, as well as overall quality of life. They now have a new option that may help improve their hearing by amplifying sounds over a broad spectrum of frequencies,” said William Maisel, M.D., M.P.H., deputy director for science and chief scientist in the FDA’s Center for Devices and Radiological Health.
A study with the new device showed a 33% improvement in word recognition after 30 days of use. The company in a press release revealed that there was also a functional benefit of 30.5 decibels on average in the high frequency range, with an average of 30-40 dB of functional benefit noted at 6,000 Hz and above and a maximum of 68 dB at 9,000-10,000 Hz.
Source-Medindia