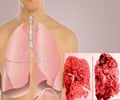
The response rate and duration of response for Keytruda were much greater than for drugs traditionally used to treat non-small cell lung cancer.
Richard Pazdur, director of the office of hematology and oncology products in the FDA's Center for Drug Evaluation and Research, "Our growing understanding of underlying molecular pathways and how our immune system interacts with cancer is leading to important advances in medicine. Today's approval of Keytruda gives physicians the ability to target specific patients who may be most likely to benefit from this drug."
Keytruda is marketed by Merck & Co., based in Whitehouse Station, New Jersey. It was approved under FDA's accelerated approval program that provides earlier patient access to promising new drugs while the company conducts confirmatory clinical trials. The drug's safety was tested on approximately 500 patients with non-small cell lung cancer.
Edward Garon, the study's principal investigator and a researcher at University of California, Los Angeles (UCLA), said, "Because so many of the patients in the study showed significant long-lasting responses, in October 2014 the FDA granted the drug breakthrough therapy status for use in lung cancer, allowing it to be fast-tracked for approval. The approval of this drug and a test to identify patients most likely to benefit has the potential to transform the way that lung cancer is treated."
Garon further added, "The quality and duration of disease response that was seen in the trial had previously been extremely rare in lung cancer. For people battling this deadly disease, this approach provides real hope of long-lasting responses while avoiding the toxicities of typical chemotherapy."
The response rate and duration of response for Keytruda were much greater than for drugs traditionally used to treat NSCLC. In the three-year clinical trial, the overall response rate was 19%.
Advertisement